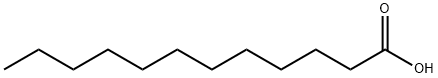
라우르산
|
|
라우르산 속성
- 녹는점
- 44-46 °C (lit.)
- 끓는 점
- 225 °C/100 mmHg (lit.)
- 밀도
- 0.883 g/mL at 25 °C (lit.)
- 증기압
- 1 mm Hg ( 121 °C)
- FEMA
- 2614 | LAURIC ACID
- 굴절률
- 1.4304
- 인화점
- >230 °F
- 저장 조건
- 2-8°C
- 용해도
- 4.81mg/L
- 물리적 상태
- 플레이크의 결정성 분말
- 산도 계수 (pKa)
- pKa 4.92(H2O,t =25.0) (Uncertain)
- Specific Gravity
- 0.883
- 색상
- 하얀색
- 냄새
- 100.00%에서. 순한 지방 코코넛 베이 오일
- ?? ??
- 지방이 많은
- 폭발한계
- 0.6%(V)
- 수용성
- 불용성
- 최대 파장(λmax)
- 207nm(MeOH)(lit.)
- Merck
- 14,5384
- JECFA Number
- 111
- BRN
- 1099477
- 안정성
- 안정적인. 타기 쉬운. 염기, 산화제, 환원제와 호환되지 않습니다.
- InChIKey
- POULHZVOKOAJMA-UHFFFAOYSA-N
- LogP
- 5
- 해리상수
- 5.3 at 20℃
- CAS 데이터베이스
- 143-07-7(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/38-41-36/37/38 | ||
| 안전지침서 | 37/39-26-39-36-36/39-24/25 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | OE9800000 | ||
| 자연 발화 온도 | 250 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29159010 | ||
| 유해 물질 데이터 | 143-07-7(Hazardous Substances Data) | ||
| 독성 | LD50 i.v. in mice: 131 ±5.7 mg/kg (Or, Wretlind) | ||
| 기존화학 물질 | KE-12855 |
라우르산 C화학적 특성, 용도, 생산
물성
도데실산 또는 도데칸산이라고도 한다. 백색 침상결정(針狀結晶) 또는 분말 로 녹는점 43.5 ℃, 끓는점 298.9 ℃, 비중 0.8690(50 ℃)이다. 글리세리드로서 야자유 속에 존재한 다. 물에는 녹지 않지만, 벤젠 등에는 잘 녹는다.용도
명칭은 월계수의 학명에서 유래되었다. 세제 ·표 면활성제의 원료로 사용하는 라우릴알코올의 원료가 된다.순도시험
(1) 산가 : 이 품목 0.5g을 정밀히 달아 유지류시험법 중 산가에 따라 시험할 때, 252~287이어야 한다.
(2) 응고점 : 이 품목의 응고점은 26.0~44.0℃이어야 한다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 요오드가 : 이 품목 약 8.3g을 정밀히 달은 다음 미리 빙초산․시클로헥산의 혼액(1 : 1) 20mL 및 위이스시액 25mL를 넣어 둔 500mL 공전삼각플라스크에 가해주고 마개를 하고 격렬히 흔들어 준 다음 1시간 어두운 곳에 방치시킨 후 요오드칼륨시액 20mL, 끓여서 식힌 물 100mL를 가하여 과량의 요오드를 0.1N 치오황산나트륨용액으로 적정한다. 이때, 황색이 거의 없어질 때까지 계속 흔들어 주면서 일정하게 0.1N 치오황산나트륨용액을 적가한 다음 다시 전분시액을 가하여 청색이 완전히 없어질 때까지 적정을 계속한다. 종말점 가까이에서는 마개를 하여 격렬히 흔들어 준다. 따로 같은 방법으로 공시험을 행하고 다음 계산식에 따라 요오드가를 구할 때, 그 양은 3.0 이하이어야 한다.
B : 공시험의 0.1N 치오황산나트륨용액의 소비량(mL)
S : 본시험의 0.1N 치오황산나트륨용액의 소비량(mL
(7) 검화가 : 이 품목 3g을 정밀히 달아 250mL 플라스크에 넣고 0.5N 알콜성수산화칼륨용액 50mL를 가한 액을 시험용액으로 하여 유지류시험법 중 검화가에 따라 시험하였을 때, 그 값은 253~287이어야 한다.
(8) 불검화물 : 이 품목 5g을 정밀히 달아 250mL 플라스크에 취하고 수산화칼륨 2g 및 에탄올 40mL를 가해주고 환류냉각기를 부착한 다음 조용히 1시간 가열한다. 플라스크의 내용물을 40mL, 80mL, 130mL 눈금이 표시되어 있는 분액여두(길이 30cm, 직경 3.5cm)에 옮기고 플라스크는 충분한 양의 에탄올로 씻어 분액여두에 합하여 40mL로 하고, 다시 더운물과 찬물을 사용하여 플라스크를 씻어 옮겨주고 전량을 80mL로 한다. 마지막으로 석유에테르 수 mL로 플라스크를 씻어 분액여두에 옮겨주고 식힌 다음 석유에테르 50mL를 가해주고 실온이 되도록 내용물을 식힌 후 마개를 하고 최소한 1분간 격렬히 진탕한 후 방치하여 두 층으로 분리시킨다. 가능한 한 완전히 분리하고 상층액인 에테르층을 500mL 분액여두에 모은 다음 석유에테르 50mL씩으로 6번 추출한 후 추출액을 처음의 추출액과 합치고 이를 10% 에탄올 25mL로 씻어 주고 물층이 페놀프탈레인시액으로 정색하지 않을 때까지 이 조작을 반복한 후 물층을 완전히 제거한다. 에테르추출액은 미리 무게를 달아둔 비이커에 옮기고 에테르 10mL를 사용하여 분액여두를 씻은 다음 비이커에 합한다. 증기욕상에서 비이커의 에테르를 증발건고 한 다음 100℃에서 30분간 항량이 될 때까지 건조하고, 데시케이터내에서 방냉하여 잔류량을 평량한다. 이어서 잔류물을 미리 페놀프탈레인시액을 지시약으로 하여 수산화나트륨시액으로 중화시킨 따뜻한 알콜 50mL에 녹이고 0.02N 수산화나트륨용액으로 엷은 홍색이 지속될 때까지 적정하고 그 소비 mL수에 5.659(mg)를 곱하여 올레인산으로서의 양을 구하고 다음 계산식에 따라 불검화물 값을 구할 때, 그 양은 0.3% 이하이어야 한다.
정의
이 품목은 코코넛오일 및 기타 식물성지방에서 얻어지는 고형지방산으로서 그 주성분은 라우린산(C12H24O2)이다.
강열잔류물
이 품목 10g을 취하여 강열잔류물시험법에 따라 시험할 때, 그 양은 0.1% 이하이어야 한다.
개요
Lauric acid, also known as dodecanoic acid, is a saturated fatty acid with a 12-carbon atom chain, thus falling into the medium chain fatty acids, is a white crystalline carboxylic acid with a faint odor of bay oil or soap. It has been found at high levels in coconut oil. Lauric acid induces the activation of NF-κB and the expression of COX-2, inducible nitric oxide synthase (iNOS), and IL-1α in RAW 264.7 cells when used at a concentration of 25 μM.물리적 성질
Lauric acid occurs as a white crystalline powder with a slight odor of bay oil or a fatty odor. It is a common constituent of most diets; large doses may produce gastrointestinal upset.화학적 성질
Like many other fatty acids, lauric acid is inexpensive, has a long shelf-life, and is non-toxic and safe to handle. It is mainly used for the production of soaps and cosmetics. For these purposes, lauric acid is neutralized with sodium hydroxide to give sodium laurate, which is a soap. Most commonly, sodium laurate is obtained by saponification of various oils, such as coconut oil. These precursors give mixtures of sodium laurate and other soaps.출처
Lauric acid, as a component of triglycerides, comprises about half of the fatty acid content in coconut oil, laurel oil, and in palm kernel oil (not to be confused with palm oil) , Otherwise it is relatively uncommon. It is also found in human breast milk ( 6.2 % of total fat), cow's milk (2.9%), and goat's milk (3.1 %).용도
Given its foaming properties, the derivatives of lauric acid (h-dodecanoic acid) are widely used as a base in the manufacture of soaps, detergents, and lauryl alcohol. Lauric acid is a common constituent of vegetable fats, especially coconut oil and laurel oil. It may have a synergistic effect in a formula to help fight against mircoorganisms. It is a mild irritant but not a sensitizer, and some sources cite it as comedogenic.생산 방법
Lauric acid is a fatty carboxylic acid isolated from vegetable and animal fats or oils. For example, coconut oil and palm kernel oil both contain high proportions of lauric acid. Isolation from natural fats and oils involves hydrolysis, separation of the fatty acids, hydrogenation to convert unsaturated fatty acids to saturated acids, and finally distillation of the specific fatty acid of interest.정의
ChEBI: Lauric acid is a straight-chain, twelve-carbon medium-chain saturated fatty acid with strong bactericidal properties; the main fatty acid in coconut oil and palm kernel oil. It has a role as a plant metabolite, an antibacterial agent and an algal metabolite. It is a straight-chain saturated fatty acid and a medium-chain fatty acid. It is a conjugate acid of a dodecanoate. It derives from a hydride of a dodecane.일반 설명
White solid with a slight odor of bay oil.공기와 물의 반응
Insoluble in water.건강위험
May be harmful by inhalation, ingestion or skin absorption. Vapor or mist is irritating to eyes, mucous membrane and upper respiratory tract. Causes eye and skin irritation.화재위험
Behavior in Fire: May cause dust explosion.Pharmaceutical Applications
pharmaceutical applications it has also been examined for use as an enhancer for topical penetration and transdermal absorption, rectal absorption, buccal delivery,(14) and intestinal absorption. It is also useful for stabilizing oil-in-water emulsions. Lauric acid has also been evaluated for use in aerosol formulations.Safety
Lauric acid is widely used in cosmetic preparations, in the manufacture of food-grade additives, and in pharmaceutical formulations. General exposure to lauric acid occurs through the consumption of food and through dermal contact with cosmetics, soaps, and detergent products. Lauric acid is toxic when administered intravenously.Occupational exposure may cause local irritation of eyes, nose, throat, and respiratory tract, although lauric acid is considered safe and nonirritating for use in cosmetics. No toxicological effects were observed when lauric acid was administered to rats at 35% of the diet for 2 years. Acute exposure tests in rabbits indicate mild irritation. After subcutaneous injection into mice, lauric acid was shown to be noncarcinogenic.
LD50 (mouse, IV): 0.13 g/kg
LD50 (rat, oral): 12 g/kg
Carcinogenicity
Lauric acid was not carcinogenic in the BALB/c:CFW mouse after repeated subcutaneous injections. Lauric acid applied twice weekly for 20 weeks did not promote tumors in mice initiated with 9,10- dimethyl-1,2-benzanthracene. After more extended application (daily, 6 days/week, for 31 weeks), lauric acid caused an increase in skin papillomas, but no histologically malignant tumors were found. Lauric acid was not carcinogenic in rats after exposure in the diet to 35% lauric acid for 2 years.저장
Lauric acid is stable at normal temperatures and should be stored in a cool, dry place. Avoid sources of ignition and contact with incompatible materials.Purification Methods
Distil the acid in a vacuum. Also crystallise it from absolute EtOH, or from acetone at -25o. Alternatively, purify it via its methyl ester (b 140.0o/15mm), as described for capric acid. It has also been purified by zone melting. [cf Beilstein 1 III 2913.]비 호환성
Lauric acid is incompatible with strong bases, reducing agents, and oxidizing agents.Regulatory Status
GRAS listed. Lauric acid is listed as a food additive in the EAFUS list compiled by the FDA. Reported in the EPA TSCA Inventory.라우르산 준비 용품 및 원자재
원자재
준비 용품
라우르산 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 |
admin@hbsaisier.cn | China | 747 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@yibangte.com | China | 2987 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 2259 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| City Chemical LLC | 2039322489 |
sales@citychemical.com | United States | 168 | 58 |