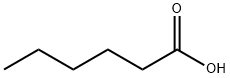
핵산산
|
|
핵산산 속성
- 녹는점
- -4 °C (lit.)
- 끓는 점
- 202-203 °C (lit.)
- 밀도
- 0.927 g/mL at 25 °C (lit.)
- 증기 밀도
- 4 (vs air)
- 증기압
- 0.18 mm Hg ( 20 °C)
- FEMA
- 2559 | HEXANOIC ACID
- 굴절률
- n
20/D 1.4161(lit.)
- 인화점
- 220 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 물: 약간 녹는다1.082g/100g(lit.)
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 4.85(at 25℃)
- Specific Gravity
- 0.929 (20/4℃)
- 색상
- 무색투명~연황색
- 수소이온지수(pH)
- 3.95(1 mM solution);3.43(10 mM solution);2.93(100 mM solution);
- 냄새
- 프로필렌 글리콜 중 10.00%. 신 지방 땀 치즈
- ?? ??
- 지방이 많은
- Odor Threshold
- 0.0006ppm
- 수용성
- 1.1g/100mL(20℃)
- Merck
- 14,1759
- JECFA Number
- 93
- BRN
- 773837
- Dielectric constant
- 2.6(71℃)
- 안정성
- 안정적인. 염기, 환원제 및 산화제와 호환되지 않습니다. 가연성.
- InChIKey
- FUZZWVXGSFPDMH-UHFFFAOYSA-N
- LogP
- 1.92 at 25℃
- CAS 데이터베이스
- 142-62-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-21-20/21/22 | ||
| 안전지침서 | 26-36/37/39-45-25 | ||
| 유엔번호(UN No.) | UN 2829 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | MO5250000 | ||
| F 고인화성물질 | 13 | ||
| 자연 발화 온도 | 380 °C | ||
| 위험 참고 사항 | Irritant | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 29159000 | ||
| 유해 물질 데이터 | 142-62-1(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 3.0 g/kg (Smyth, Carpenter) | ||
| 기존화학 물질 | KE-19797 |
핵산산 C화학적 특성, 용도, 생산
개요
Hexanoic acid (also known as Caproci acid), is the carboxylic acid derived from hexane with the general formula C5H11COOH. It is a colorless oily liquid with an odor that is fatty, cheesy, waxy, and like that of goats or other barnyard animals. It is a fatty acid found naturally in various animal fats and oils, and is one of the chemicals that give the decomposing fleshy seed coat of the ginkgo its characteristic unpleasant odor. The primary use of hexanoic acid is in the manufacture of its esters for artificial flavors, and in the manufacture of hexyl derivatives, such as hexylphenols. Hexanoic acid belongs to medium chain triglycerides (MCT) that are widely used as a nutrition supplement that added to foods, drugs and cosmetics.화학적 성질
Hexanoic acid is a colourless liquid that has a sickening, sweaty, rancid, sour, sharp, pungent, cheesy, fatty, unpleasant odor reminiscent of copra oil. It exhibits an acrid taste. Hexanoic acid may be prepared by fractionation of the volatile fatty acids of coconut oil.물리적 성질
Hexanoic acid is oily, colorless or slightly yellow, and liquid at room temperature. Odor is that of Limburger cheese. Soluble in alcohol and ether; slightly soluble in water. It is derived from the crude fermentation of butyric acid; or by fractional distillation of natural fatty acids.출처
Hexanoic acid is found in many foods, some of which are tapioca pearl, meat bouillon, pecan nut, and oval-leaf huckleberry. A secondary product of butyric fermentation; reported found in the essential oils of lavender, camphor, palmarosa, lemongrass and Juniperus phoenicea; in a few fruital aromas: apple, currant and strawberry; also identified among the constituents of petitgrain lime oil.용도
Hexanoic acid is used to prepare esters by reacting with alcohols, which finds application in artificial flavors. It is also involved in the production of hexylphenols, hexanoates and caproates. Further, it is used as non-viral gene carrier as well as to protect tomato plants from Botrytis cinerea.정의
ChEBI: Hexanoic acid is a C6, straight-chain saturated fatty acid. It has a role as a human metabolite and a plant metabolite. It is a straight-chain saturated fatty acid and a medium-chain fatty acid. It is a conjugate acid of a hexanoate.제조 방법
hexanoic acid is produced by fractionation of the volatile fatty acids of coconut oil.일반 설명
Hexanoic acid is an oily carboxylic acid found (as glycerides) in cow’s milk and some vegetable oils. It is a white crystalline solid or colorless to light yellow solution with an unpleasant odor. Insoluble to slightly soluble in water and less dense than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make perfumes.공기와 물의 반응
Slightly water soluble.위험도
Strong irritant to tissue.건강위험
Harmful if swallowed, inhaled, or absorbed through skin. Material is extremely destructive to tissue of mucous membranes and upper respiratory tract, eyes and skin. Inhalation may be fatal as a result of spasm, inflammation and edema of the larynx and bronchia, chemical pneumonitis and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting.화재위험
Special Hazards of Combustion Products: Irritating vapor may be generated.Purification Methods
Dry the acid with MgSO4 and fractionally distil it from CaSO4. [Beilstein 2 IV 917.]핵산산 준비 용품 및 원자재
원자재
준비 용품
헥산아마이드
프로필 헥산산
에틸 헵타노에이트
cis-3-Hexenyl hexanoate
노르말펜틸아민
1,2-Phenylenediacetic acid
헥실레소시놀
카프로일클로라이드
HEXANOIC ANHYDRIDE
Bupivacaine
Cyclohexanehexanoic acid, 2-propenyl ester
카프로산에틸
PENTYL HEXANOATE
카프롤릭산부틸에스테르
카프로익산메틸에스테르
DL-2-Bromohexanoic acid
Ethyl1-butyl-2-piperidinecarbxylate
헥사놀(1-)
ISOAMYL HEXANOATE
펜타논-2
카프로산 알릴
Isobutyl hexanoate
카프론알데히드
핵산산 공급 업체
글로벌( 480)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Xiangduo Industry Co., Ltd. | +86-15981848961 +86-15981848961 |
sales@xiangduochem.com | China | 497 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shochem(Shanghai) Co.,Ltd | 86-21-50800795 |
info@shochem.com | CHINA | 288 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |