다이에틸렌 글리콜 다이메틸 에테르
|
|
다이에틸렌 글리콜 다이메틸 에테르 속성
- 녹는점
- -64 °C (lit.)
- 끓는 점
- 162 °C (lit.)
- 밀도
- 0.944 g/mL at 20 °C (lit.) 0.939 g/mL at 25 °C (lit.)
- 증기 밀도
- 4.6 (vs air)
- 증기압
- 3 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.408(lit.)
- 인화점
- 134.6 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 클로로포름(소량으로 용해), 에틸아세테이트(약간 용해됨), 메탄올(약간 용해됨)
- 물리적 상태
- 액체
- 색상
- ≤10(APHA)
- 냄새
- 가벼운 미묘한.
- 상대극성
- 0.244
- 폭발한계
- 1.4-17.4%(V)
- 수용성
- 혼용 가능
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 225 nm Amax: 1.00
λ: 240 nm Amax: 0.50
λ: 260 nm Amax: 0.20
λ: 280 nm Amax: 0.08
λ: 320-400 nm Amax: 0.01
- Merck
- 14,3165
- BRN
- 1736101
- Dielectric constant
- 7.2999999999999998
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제와 호환되지 않습니다. 공기나 빛에 민감할 수 있습니다.
- InChIKey
- SBZXBUIDTXKZTM-UHFFFAOYSA-N
- LogP
- -0.36 at 25℃
- CAS 데이터베이스
- 111-96-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 60-61-10-19 | ||
| 안전지침서 | 53-45 | ||
| 유엔번호(UN No.) | UN 3271 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | KN3339000 | ||
| F 고인화성물질 | 3-10-23 | ||
| 자연 발화 온도 | 370 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29091990 | ||
| 유해 물질 데이터 | 111-96-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 4760 mg/kg | ||
| 기존화학 물질 | KE-27705 | ||
| 중점관리물질 필터링 | 별표1-65 |
다이에틸렌 글리콜 다이메틸 에테르 C화학적 특성, 용도, 생산
개요
Bis (2-methoxyethyl) ether, also known as diglyme, is a linear aliphatic diether widely used as a solvent and present as a clear liquid at room temperature with a mild ether odor. The compound is notknown to occur in nature. It is synthesized from ethylene oxide and methanol in the presence of either acidic or basic catalysts. The reaction is based on the classic Williamson ether synthesis. It can also be produced from diethylene glycol and dimethyl sulfate. In June 2012, ECHA proposed addition of diglyme to the REACH very high concern list.화학적 성질
Diethylene glycol dimethyl ether is a clear, water-white neutral liquid of faint, pleasant odor. This ether may be used as a solvent for alkali metal hydrides for use in such reactions as reduction, alkylation and condensation. It may also be used as a lacquer solvent.용도
Diethylene glycol dimethyl ether is used as a solvent in organic reactions due to its stability towards higher pH and its high boiling point. It is particularly involved in reactions utilizing organometallic reagents such as Grignard reactions and metal hydride reductions. It is also a solvent for hydroboration reactions with diborane.정의
ChEBI: A polyether that is the dimethyl ether derivative of diethylene glycol.일반 설명
Colorless watery liquid with a pleasant odor. Floats and mixes with water.공기와 물의 반응
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. A mixture of liquid air and diethyl ether exploded spontaneously, [MCA Case History 616(1960)]. Water soluble.반응 프로필
A violent explosion occurred when lithium aluminum hydride was being used to dry 2-Methoxyethyl ether. The ignition may have occurred due to the presence of large amounts of water or perhaps peroxide formed in the ether. About 75% of the ether had been removed when the explosion occurred, [MCA Case History 1494 (1968)].건강위험
INGESTION (severe cases): nausea, vomiting, abdominal cramps, weakness progressing to coma.화재위험
2-Methoxyethyl ether is combustible.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Synthesis
265 g (2.5 mol) of diethylene glycol, 320 g (10.0 mol) of methanol, and 14.3 g (0.0125 equivalents) of NAFION 1100 EW Polymer (H+ form) were charged to a one-liter autoclave. After sealing and pressure testing, the contents of the autoclave were agitated, and the autoclave was pressurized to 100 psi with nitrogen. After 5 minutes of agitation, the autoclave was depressurized. This process was repeated two more times to ensure complete deoxygenation. After deoxygenation, the autoclave was heated to a temperature of 198 ℃, and the contents of the autoclave were agitated at 1900 rpm for 5 hours at temperature (198-200 ℃). A pressure of 810 psi was obtained. After 5 hours, the autoclave was cooled and sampled. By analysis, a total of 77.2% by weight of the diethylene glycol (1.93 moles) was converted in the reaction, producing 0.335 mol of Diglyme. The co-product includes 1,4 dioxane and the intermediate diethylene glycol monomethyl ether.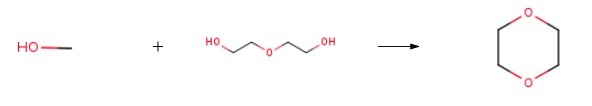
환경귀착
The metabolite 2-methoxyacetic acid, which is generated from 2-methroxyethanol by the reaction of alcohol dehydrogenase, may be important for the toxic effects. It can undergo activation to methoxyacetyl coenzyme A and enter the Krebs cycle or fatty acid biosynthesis. Several metabolites of 2-methoxyethanol, such as 2-methoxy-N-acetyl glycine, have been identified that support this pathway. Thus, 2-methoxyacetic acid may interfere with essential metabolic pathways of the cell, and it was hypothesized that this causes the testicular lesions and malformations in experimental animals.Purification Methods
Dry diglyme with NaOH pellets or CaH2, then reflux with, and distil (under reduced pressure) it from Na, CaH2, LiAlH4, NaBH4 or NaH. These operations are carried out under N2. The amine-like odour of diglyme has been removed by shaking with a weakly acidic ion-exchange resin (Amberlite IR-120) before drying and distilling. Addition of 0.01% NaBH4 to the distillate inhibits peroxidation. Purify it also as for dioxane. It has been passed through a 12-in column of molecular sieves to remove water and peroxides. [Beilstein 1 IV 2393.]다이에틸렌 글리콜 다이메틸 에테르 준비 용품 및 원자재
원자재
디에틸렌 글리콜 모노메틸 에테르
Diethanol
수산화나트륨
디메틸설페이트
Ethane, 1,1,1-trifluoro-2-(2-methoxyethoxy)-
BIS(2,2,2-TRIFLUOROETHYL) ETHER
2-메톡시에틸-p-톨루엔 설포네이트
2,2,2-TRIFLUOROETHYL P-TOLUENESULFONATE
2-Iodo-1,1,1-trifluoroethane
준비 용품
5-AMINOMETHYL-PYRROLIDIN-2-ONE
2-BROMO-6-(1H-PYRAZOL-1-YL)PYRIDINE
1-(2-Methoxyphenyl)piperazine hydrobromide
(2,5-DIMETHYL-1,3-OXAZOL-4-YL)METHANOL
6,6'-Dimethyl-2,2'-dipyridyl
5,5'-DIMETHYL-2,2'-DIPYRIDYL
2-METHYLCYCLOPROPANECARBOXYLIC ACID
2,5-DIMETHYL-1,3-OXAZOLE-4-CARBALDEHYDE
3-CYCLOPENTENE-1-OL
사이클로헥센옥사이드
1-(1-NAPHTHYL)PIPERAZINE HYDROCHLORIDE
메틸알콜
펜타씬
다이에틸렌 글리콜 다이메틸 에테르 공급 업체
글로벌( 554)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 |
marketing1@neostarunited.com | China | 8349 | 58 |
| Anhui lixing chemical co.,ltd | +86-5638152626 +86-17756305689 |
gdq@lixingchem.com | China | 10 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 |
sales@tjzxchem.com | China | 559 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |








