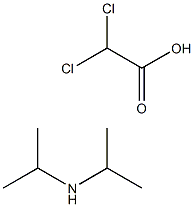
Diisopropylammonium dichloroacetate
|
|
Diisopropylammonium dichloroacetate 속성
- 녹는점
- 119-121°
- RTECS 번호
- AG6475000
- 저장 조건
- Sealed in dry,Room Temperature
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨)
- 물리적 상태
- 결정성 분말
- 색상
- 하얀색
- Merck
- 14,3197
- 안정성
- 흡습성
- InChI
- InChI=1S/C6H15N.C2H2Cl2O2/c1-5(2)7-6(3)4;3-1(4)2(5)6/h5-7H,1-4H3;1H,(H,5,6)
- InChIKey
- ILKBHIBYKSHTKQ-UHFFFAOYSA-N
- SMILES
- C(O)(=O)C(Cl)Cl.CC(NC(C)C)C
- CAS 데이터베이스
- 660-27-5
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| TSCA | Yes | ||
|---|---|---|---|
| HS 번호 | 2921199990 | ||
| 독성 | LD50 orally in mice: 1700 mg/kg (Kraushaar) |
Diisopropylammonium dichloroacetate C화학적 특성, 용도, 생산
개요
Diisopropylammonium dichloroacetate is a hepatoprotective drug that improves the energy metabolism of hepatocytes, promotes the regeneration of injured hepatocytes, increases the rate of tissue cell respiration and oxygen respiration, and reduces the accumulation of fat in the liver. It is mainly used clinically for the treatment of fatty liver, intrahepatic cholestasis, and general liver dysfunction. It is also used in the treatment of acute and chronic hepatitis, hepatomegaly, and early cirrhosis.화학적 성질
White or off-white loose mass or powder, slightly bitter, easily soluble in water, ethanol or chloroform, slightly soluble in ether.역사
In the 1950s, the compound diisopropylammonium dichloroacetate (DIPA) was used in the synthesis of methylated derivatives of a purportedly naturally occurring B vitamin (pangamic acid; dgluconodimethylaminoacetate). Anecdotal clinical reports appeared claimingefficacy in various metabolic and cardiovascular disorders from pharmaceuticalmixtures of pangamic acid and DIPA. In 1970,DCA was identified as themetabolically active moiety of DIPA(Stacpoole & Felts,1970) and it has beenused thereafter almost exclusively as the sodium salt.용도
Diisopropylammonium dichloroacetate (DIPA) is known to produce a significant and prolonged hypoglycemic effect in alloxan-diabetic but not in normal rats.Diisopropylamine 2,2-Dichloroacetate is used in the treatment of antituberculosis drugs-induced liver injury.
정의
ChEBI: Diisopropylamine dichloroacetate is an organohalogen compound and a carboxylic acid.Synthesis
Diisopropylammonium dichloroacetate is prepared by the reaction of dichloro-acetic acid and diisopropylamine. The steps are as follows:1 volume of acetone is mixed with 1.5 volumes of cyclohexane to obtain a mixed solvent; Add 1 mol of diisopropylamine (101.19g, C6H15N, Mr=101.19) into 150ml of mixed solvent, stir evenly, and heat the liquid material to 50°C; slowly add 1mol of dichloroacetic acid (128.94g, C2H2Cl2O2, Mr=128.94) dropwise to the liquid material obtained in step (at a speed of about 10g/min) under heat preservation and stirring conditions,After the feeding is complete, keep warm and continue to stir for 3 hours, then naturally cool to 4-8°C, and then stand at this temperature for 10 hours; Filter the material obtained in step, separate the mother liquor, collect white crystals, and dry at 70° C. to obtain 216.8 g of the diisopropylamine dichloroacetate (C8H17Cl2NO2, Mr=230.13), with a molar yield of 94.2%.
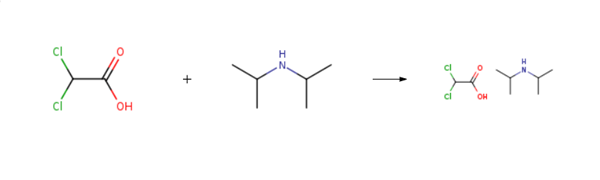
Diisopropylammonium dichloroacetate 준비 용품 및 원자재
원자재
준비 용품
Diisopropylammonium dichloroacetate 공급 업체
글로벌( 258)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 |
sales@sdperfect.com | China | 294 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 |
sales@frappschem.com | China | 885 | 50 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 |
sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9355 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32836 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Jinan Shengqi pharmaceutical Co,Ltd | 86+18663751872 |
christine@shengqipharm.com | CHINA | 491 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
Diisopropylammonium dichloroacetate 관련 검색:
다이클로로메탄 타이타늄 다이옥사이드 알파-톨루엔올 다이아이소프로필아민 이소프로필아민
Granisetron
Armillarisin A
Omeprazole
Glutathione
Tropisetron
Pantoprazole
Clotrimazole
L-Ornithine L-aspartate salt
Dibutyryl adenosine cyclophosphate calcium
MUCO-INOSITOL
Diisopropylammonium dichloroacetate
(5S)-(-)-2,2,3-TRIMETHYL-5-BENZYL-4-IMIDAZOLIDINONE DICHLOROACETIC ACID
(5R)-(+)-2,2,3-TRIMETHYL-5-BENZYL-4-IMIDAZOLIDINONE DICHLOROACETIC ACID