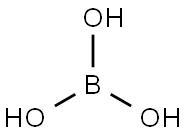
붕산
|
|
붕산 속성
- 녹는점
- 160 °C (dec.) (lit.)
- 끓는 점
- 219-220 °C (9.7513 mmHg)
- 밀도
- 1.440 g/cm3
- 증기압
- 2.6 mm Hg ( 20 °C)
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 용해성
- 물리적 상태
- 해결책
- 산도 계수 (pKa)
- 8.91±0.43(Predicted)
- Specific Gravity
- 1.435
- 색상
- ≤10(APHA)
- 냄새
- 냄새 없는
- pH 범위
- 3.8 - 4.8
- 수소이온지수(pH)
- 3.6-4.4 (25℃, saturated solution in H2O)
- 수용성
- 49.5g/L(20℃)
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.05
λ: 280 nm Amax: 0.05
- Merck
- 14,1336
- BRN
- 1697939
- 노출 한도
- ACGIH: TWA 2 mg/m3; STEL 6 mg/m3
- InChIKey
- KGBXLFKZBHKPEV-UHFFFAOYSA-N
- LogP
- -1.09 at 22℃
- CAS 데이터베이스
- 10043-35-3(CAS DataBase Reference)
- NIST
- B(OH)3(10043-35-3)
안전
- 위험 및 안전 성명
| 위험품 표기 | Xi,T,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-60-63-62-61 | ||
| 안전지침서 | 26-36-53-45-37/39-36/37/39-22-24/25-23 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | ED4550000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 28100090 | ||
| 유해 물질 데이터 | 10043-35-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 5.14 g/kg (Smyth). | ||
| 기존화학 물질 | KE-03499 | ||
| 유해화학물질 필터링 | 2019-1-942 | ||
| 중점관리물질 필터링 | 별표1-144 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 붕산[Boric acid; 10043-35-3, 11113-50-1] 및 이를 0.3% 이상 함유한 혼합물 |
붕산 C화학적 특성, 용도, 생산
물성
붕산은 흰색을 띄고 냄새가 없다. 비중은 15℃에서 1.435, 용해도는 30℃에서 6.35% (물) 고온 알코올, 글리세롤에는 용해가 잘 되며, 아세톤과 에테르에는 약간 용해된다.개요
붕산은 무색의 결정체 혹은 백색 분말의 형태로 존재하고 물에 용해된다. 광물로 존재할 때는 붕산석이라고 부른다. 붕산은 주로 붕산염 광물을 황산과 반응시켜 생산한다. 붕산이나 붕산염은 바닷물에서 발견된다. 또한 식물과 거의 모든 과일에도 존재한다고 알려져 있다. 일부 화산 지역에서는 유리산이 천연 상태로 발견된다. 또한 유리산은 많은 광물(붕사, 방붕석, 보로나트로카이사이트 및 회붕석)에도 성분으로 포함되어 있다.용도
붕산은 칸디다증과 무좀과 같은 효모 및 곰팡이감염의 치료에 소독제로 사용되고, 내후성 목재와 내화성 직물에 사용된다. 또한 시멘트, 질그릇, 자기제품, 법랑 세공품, 유리, 붕산염, 피혁, 카펫트, 모자, 비누, 인조 보석의 제작에서 보존제로 사용되고 니켈도금조, 인쇄와 염색, 도색, 사진에 사용된다. 심지 제조에도 사용된다. 전기 콘덴서와 경화 강철에 사용되고 바퀴벌레 살충제로도 사용된다. 또한 붕산은 색조 화장품, 피부 및 모발 관리 제품, 데오도란트, 보습 크림, 구취 제거제, 면도 크림 등 수많은 화장품에서 최대 5% 농도로 사용된다.독성
붕산은 위장관, 장막강, 찰과되거나 염증이 있는 피부에 쉽게 흡수된다. 손상되지 않은 피부에는 흡수되지 않는다. 투여량의 약 50%가 24시간 내에 배출된다. 만성 투여 시에도 2주 후면 소변 배출이 평탄한 상태에 도달한다. 뇌, 간, 신장에 붕산이 다량 축적된다.화학적 성질
White powder or granules and odorless. It is incompatible with potassium, acetic anhydride, alkalis, carbonates, and hydroxides. Boric acid has uses in the production of textile fiberglass, flat panel displays, and eye drops. Boric acid is recognized for its application as a pH buffer and as a moderate antiseptic agent and emulsifier.물리적 성질
Colorless, transparent triclinic crystal or white granule or powder; density 1.435 g/cm3; melts at 171°C under normal heating; however, slow heating causes loss of water; sparingly soluble in cold water (4.7% at 20°C); pH of 0.1M solution 5.1; readily dissolves in hot water (19.1% at 80°C and 27.5% at 100°C); also soluble in lower alcohols and moderately soluble in pyridine.용도
For weatherproofing wood and fireproofing fabrics; as a preservative; manufacture of cements, crockery, porcelain, enamels, glass, borates, leather, carpets, hats, soaps, artificial gems; in nickeling baths; cosmetics; printing and dyeing, painting; photography; for impregnating wicks; electric condensers; hardening steel. Also used as insecticide for cockroaches and black carpet beetles.생산 방법
Boric acid occurs naturally as the mineral sassolite. However, the majority of boric acid is produced by reacting inorganic borates with sulfuric acid in an aqueous medium. Sodium borate and partially refined calcium borate (colemanite) are the principal raw materials. When boric acid is made from colemanite, the fineground ore is vigorously stirred with mother liquor and sulfuric acid at about 908℃. The by-product calcium sulfate is removed by filtration, and the boric acid is crystallized by cooling the filtrate.제조 방법
Boric acid is produced from borax, colemanite, or other inorganic borates by reaction with sulfuric acid or hydrochloric acid, and cooling the solution to proper temperature:Na2B4O7 ? 10Η2Ο + H2SO4 → 4H3BO3 + Na2SO4 + 5H2O
It also may be prepared by extraction of weak borax brine with a kerosene solution of an aromatic diol, such as 2-ethyl-1,3-hexanediol or 3-chloro- 2-hydroxy-5-(1,1,3,3-tetramethylbutyl)benzyl alcohol. The diol-borate chelate formed separates into a kerosene phase. Treatment with sulfuric acid yields boric acid which partitions into aqueous phase and is purified by recrystallization.
World Health Organization (WHO)
Boric acid and some borates were formerly extensively used as disinfectants and antiinflammatory agents. By the late 1960s an association between the death of many infants and application of high concentrations of boric acid contained in topical preparations used in the treatment of napkin rash had been established. This led to the restriction of the use of boric acid in pharmaceutical preparations by many regulatory authorities. In some countries it is now permitted only as an ingredient in ophthalmological preparations.일반 설명
Boric acid is a weak monobasic acid, it accepts OH- ions, hence is a Lewis acid. In boric acid, B is sp2 hybridized, forming a planar triangle structure. The principal oxide of boron, B2O3, is obtained as a vitreous solid by dehydration of boric acid at red heat.위험도
Toxic via ingestion. Use only weak solu- tions. Irritant to skin in dry form.Pharmaceutical Applications
Boric acid is used as an antimicrobial preservative in eye drops, cosmetic products, ointments, and topical creams. It is also used as an antimicrobial preservative in foods.Boric acid and borate have good buffering capacity and are used to control pH; they have been used for this purpose in external preparations such as eye drops.
Boric acid has also been used therapeutically in the form of suppositories to treat yeast infections. In dilute concentrations it is used as a mild antiseptic, with weak bacteriostatic and fungistatic properties, although it has generally been superseded by more effective and less toxic disinfectants.
잠재적 노출
Boric acid is a fireproofing agent for wood; a preservative, and an antiseptic. It is used in the manufacture of glass, pottery, enamels, glazes, cosmetics, cements, porcelain, borates, leather, carpets, hats, soaps; artificial gems; in tanning leather; printing, dyeing, painting, and photography.저장
Boric acid is hygroscopic and should therefore be stored in an airtight, sealed container. The container must be labeled ‘Not for Internal Use’.운송 방법
UN 3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.Purification Methods
Crystallise the acid three times from H2O (3mL/g) between 100o and 0o, after filtering through sintered glass.Dry it to constant weight over metaboric acid in a desiccator. It is steam volatile. After two recrystallisations of ACS grade. it had Ag at 0.2 ppm. Its solubility (%) in H2O is 2.66 at 0o, 4.0 at 12o and 24 at 80o. At 100o it loses H2O to form metaboric acid (HBO2). When it is heated to redness or slowly to 200o, or over P2O5 in vacuo, it dehydrates to boric anhydride (B2O3) [1303-82-6] to give a white hard glass or crystals with m ~294o.The glass softens on heating and liquefies at red heat. It is an astringent, a fungicide and an antibacterial. [McCulloch J Am Chem Soc 59 2650 1937, Kelly J Am Chem Soc 63 1137 1941, Taylor & Cole J Chem Soc 70 1926, Conti J Soc Chem Ind 44 343T 1925.]비 호환성
Boric acid decomposes in heat above 100 C, forming boric anhydride and water. Boric acid is hygroscopic; it will absorb moisture from the air. Boric acid aqueous solution is a weak acid; incompatible with strong reducing agents including alkali metals and metal hydrides (may generate explosive hydrogen gas); acetic anhydride, alkali carbonates, and hydroxides. Violent reaction with powdered potassium metal, especially if impacted. Attacks iron in the presence of moisture.폐기물 처리
Boric acids may be recovered from organic process wastes as an alternative to disposal.Regulatory Status
Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IV injections; ophthalmic preparations; (auricular) otic solutions; topical preparations). Reported in the EPA TSCA Inventory. In the UK, the use of boric acid in cosmetics and toiletries is restricted. Included in the Canadian List of Acceptable Non-medicinal Ingredients.붕산 준비 용품 및 원자재
원자재
준비 용품
구리 플루오린붕산(구리 플루오르붕산)
아연 테트라플루오로붕산염
수소화나트륨
삼산화붕소(무수붕산)
Triisopropanolamine cyclic borate
메틸글리콜레이트
6-퀴놀리닐메탄올
트리에탄올아민 보레이트
나이트로메테인
불화붕산 칼륨
Foliar-fertilizer
붕산 아연
트리부틸 보레이트
삼불화붕소아세토니트릴착물
불화붕산 나트륨
나트륨 붕소수화물
ACID BLUE 80
테트라페닐붕산 나트륨
METHYL5,5,5-TRIFLUORO-4-OXOPENTANOATE
삼불화붕소화합물
Yemianbao
붕불화수소산
칼륨보로하이드라이드
1,4-다이하이드록시안트라퀴논
6-Methoxyquinoline
헥사플루오르알루민산칼륨
1-아미노-4-하이드록시-2-페녹시안트라퀴논
불화붕산 암모늄
TRIETHANOLAMINE BORATE
질화붕소
붕산 공급 업체
글로벌( 548)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| Wuhan Aoliqisi New Material Technology Co., Ltd. | +86-13545906766; +8613545906766 |
sales@whaop.com | China | 265 | 58 |
| Wuhan Golt Biotech Co., Ltd. | +8615389281203 |
maria@goltbiotech.com | China | 980 | 58 |
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 |
admin@zhanyaobio.com | China | 2136 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12465 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-18033737140 +86-17354101231 |
sales1@hbganmiao.com | China | 253 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21666 | 55 |