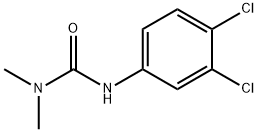
디우론
|
|
디우론 속성
- 녹는점
- 158-159°C
- 끓는 점
- 180-190°C
- 밀도
- 1.48
- 증기압
- 2(x 10-7 mmHg) at 30 °C (Hawley, 1981)
- 굴절률
- 1.5500 (estimate)
- 인화점
- 180-190°C
- 저장 조건
- 2-8°C
- 용해도
- 아세톤 중: 27℃에서 5.3wt%(Meister, 1988).
- 산도 계수 (pKa)
- -1 to -2 (quoted, Bailey and White, 1965)
- 색상
- 백색, 무취의 결정성 고체
- 수용성
- 약간 용해됨. 0.0042g/100mL
- Merck
- 14,3382
- BRN
- 2215168
- Henry's Law Constant
- 1.46(x 10-9 atm?m3/mol) at 25 °C (approximate - calculated from water solubility and vapor pressure)
- 노출 한도
- NIOSH REL: TWA 10 mg/m3.
- 안정성
- 안정적인. 강산, 강염기, 강산화제와 호환되지 않습니다.
- InChIKey
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N
- LogP
- 2.84-2.89 at 20℃ and pH4.03-9
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-40-48/22-50/53-36-20/21/22-11 | ||
| 안전지침서 | 13-22-23-37-46-60-61-2-36/37-26-16 | ||
| 유엔번호(UN No.) | UN 3077 9/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | YS8925000 | ||
| TSCA | Yes | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 29242990 | ||
| 유해 물질 데이터 | 330-54-1(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 1690 mg/kg (Boyd, Krupa) | ||
| 기존화학 물질 | KE-10186 |
디우론 C화학적 특성, 용도, 생산
개요
Diuron (330-54-1) is used as an herbicide for weed control on noncrop lands and agricultural crops such as asparagus, pineapple, cotton, and sugarcane. It is also used as a sterilant in soil, a mildewcide in paints and stains, and an algicide in fish production.화학적 성질
White, odorless crystalline solid용도
Diuron is a urea compound used as a preemergence herbicide in soils to control germinating broad-leaved grasses and weeds in crops such as apples, cotton, grapes, pears, pineapple and alfalfa; sugar cane ?owering depressant.정의
ChEBI: Diuron is a member of the class of 3-(3,4-substituted-phenyl)-1,1-dimethylureas that is urea in which both of the hydrogens attached to one nitrogen are substituted by methyl groups, and one of the hydrogens attached to the other nitrogen is substituted by a 3,4-dichlorophenyl group. It has a role as a herbicide, a photosystem-II inhibitor, a xenobiotic, an environmental contaminant and a mitochondrial respiratory-chain inhibitor. It is a dichlorobenzene and a 3-(3,4-substituted-phenyl)-1,1-dimethylurea.일반 설명
Diuron is a white crystalline solid/wettable powder and used as a herbicide. Diuron is registered for pre- and post-emergent herbicide treatment of both crop and non-crop areas, as a mildewcide and preservative in paints and stains, and as an algaecide. Diuron is a substituted urea herbicide for the control of a wide variety of annual and perennial broad-leaved and grassy weeds on both crop and non-crop sites.Thus, the application of diuron is wide for vegetation control and weed control in citrus orchards and alfalfa fields. The mechanism of herbicidal action is the inhibition of photosynthesis. Diuron was first registered in 1967. Products containing diuron are intended for both occupational and residential uses. Occupational uses include agricultural food and non-food crops; ornamental trees, flowers, and shrubs; paints and coatings; ornamental fish ponds and catfish production; and rights-of-way and industrial sites. Residential uses include ponds, aquariums, and paints.
공기와 물의 반응
Very slightly soluble in water.반응 프로필
Diuron is incompatible with the following: Strong acids .건강위험
INHALATION: May cause irritation of nose and throat. EYES: Irritation. SKIN: Moderately irritating to skin.농업용
Herbicide: Diuron is a substituted urea herbicide used to control a wide variety of annual and perennial broadleaf and grassy weeds, as well as mosses. It is used on non-crop areas and many agricultural crops such as fruit, cotton, sugar cane, alfalfa, and wheat. Diuron works by inhibiting photosynthesis. It may be found in formulations as wettable powders and suspension concentrates.상품명
330541®; AF 101®; AI3-61438®; AMETRON SC®; BOUNDRY®[C]; CHEMIURON®[C]; CEKIURON®; CRISURON®; DAILON®; DIATER®; DI-ON®; DIREX®; DITOX®; DIUMATE® Diuron; DIUREX®,[C]; DIUROL® Diuron; DIURON 4L®; DMU®; DREXEL DIURON 4L®; DROPP ULTRA®; DURAN®; DYNEX®[C] FARMCO DIURON®; FORTEX SC®; FREEFLO®; GINSTAR®; HERBURON 500 BR®; HW 920®; KARMEX®[C]; K-4®; KARMEX DIURON HERBICIDE®; KARMEX DW®; KROVAR IDF®[C]; MARMER®; STRIKER®; SUP'R FLO®; TELVAR®; TIGREX®; TREVISSIMO®; UNIDRON®; UROX D®[C]; VONDURON®Safety Profile
oneal routes. Questionable carcinogen with experimental tumorigenic and teratogenic data. Mutation data reported. When heated to decomposition it emits highly toxic fumes of Cland NOx. See also CHLOROPHENOLS.Purification Methods
Recrystallise it from 95% EtOH [Beck et al. J Am Chem Soc 108 4018 1986]. [Beilstein 12 IV 1263.]디우론 준비 용품 및 원자재
원자재
DimethylamineE
P-클로로니트로벤젠
3,4-디클로로아닐린
포스겐
염화 암모늄
2,4-디클로로-1-니트로벤젠
3,4-디클로로나이트로벤젠
Isocyanic acid 3,4-dichlorophenyl ester
준비 용품
디우론 공급 업체
글로벌( 383)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| WUXI HONOR SHINE CHEMICAL CO.,LTD | 0510-83593312 +8613665125081 |
sales@honorshinechem.com | China | 61 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 951 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |