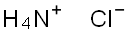염화 암모늄
|
|
염화 암모늄 속성
- 녹는점
- 340 °C (subl.) (lit.)
- 끓는 점
- 100 °C750 mm Hg
- 밀도
- 1.52
- 증기 밀도
- 1.9 (vs air)
- 증기압
- 1 mm Hg ( 160.4 °C)
- 굴절률
- 1.642
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 산도 계수 (pKa)
- 9.25[at 20 ℃]
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- Specific Gravity
- 1.53
- 수소이온지수(pH)
- 4.7 (200g/l, H2O, 25℃)(External MSDS)
- 냄새
- 냄새 없는
- ?? ??
- 암모니아의
- 수용성
- 녹는
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 260 nm Amax: ≤0.021
λ: 280 nm Amax: ≤0.019
- Merck
- 14,509
- BRN
- 4371014
- Dielectric constant
- 7(0.0℃)
- 노출 한도
- ACGIH: TWA 10 mg/m3; STEL 20 mg/m3
NIOSH: TWA 10 mg/m3; STEL 20 mg/m3
- 안정성
- 안정적인. 강산, 강염기와 호환되지 않습니다.
- CAS 데이터베이스
- 12125-02-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36-41-37/38 | ||
| 안전지침서 | 22-36-26 | ||
| 유엔번호(UN No.) | UN 3077 9 / PGIII | ||
| OEB | B | ||
| OEL | TWA: 10 mg/m3, STEL: 20 mg/m3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | BP4550000 | ||
| 자연 발화 온도 | >400 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 28271000 | ||
| 유해 물질 데이터 | 12125-02-9(Hazardous Substances Data) | ||
| 독성 | LD50 in rats (mg/kg): 30 i.m. (Boyd, Seymour); LD50 in rats (mg/kg): 1650 orally (Smeets) | ||
| 기존화학 물질 | KE-01645 |
염화 암모늄 C화학적 특성, 용도, 생산
물성
분자량은 53.50이며, 백색의 결정성 고체로 존재한다. 350°C에서 분해되며, 통제된 환경에서는 520°C에서 승화된다. 비중은 1.52이다. 물에 쉽게 용해되고, 암모니아 수용액에도 잘 용해되지만, 메탄올에는 약간 녹는다.용도
염화 암모늄은 물에 녹아 암모늄 이온과 염화 이온을 내놓으므로 물의 전기전도도를 상승시키기 때문에 건전지의 전해액으로 이용된다. 또한 염화 암모늄은 납땜시 녹은 금속이 직접 공기와 접하는 것을 막는 플럭스로 사용된다. 이 외에 직물의 착색, 가죽의 무두질, 다른 암모늄 화합물을 합성하는 데에 재료로도 사용된다.염화 암모늄은 일반적으로 비료의 질소 공급원으로는 적합하지 않다. 염화암모늄은 염소를 포함하고 있는데, 염소 성분이 토양에 축적될 경우 토양에 좋지 않은 영향을 끼치기 때문이다.
생산/준비/합성
염화 암모늄은 암모니아와 염화 수소의 중화반응으로 얻을 수 있다. 반응식은 다음과 같다.NH3 + HCl → NH4Cl
또는 수산화 암모늄과 염화 수소와의 반응으로도 얻을 수 있다. 반응식은 다음과 같다.
NH4OH + HCl → NH4Cl + H2O
반응 이후 건조, 결정화, 분리 과정을 거친다.
순도시험
(1) 용상 : 이 품목 2g을 물 20mL에 녹일 때, 그 탁도는 거의 징명 이하이어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
확인시험
이 품목은 확인시험법 중 암모늄염 및 염화물의 반응을 나타낸다.
강열잔류물
이 품목의 강열잔류물은 0.5% 이하이어야 한다.
화학적 성질
Ammonium chloride,Nl4CI, also known as ammoniae, salmiai,and ammonium nituriate,is a white crystalline solid. It is soluble in water, aqueous solutionsof ammonia, and is slightly soluble in methyl alcohol. Ammonium chloride is found in natureas a sublimation productof volcanic activity, or is produced by neutralizing HCI(either in liquid or gaseousphase) with NH3 gas or liquid NH40H then evaporating the excess H20. The salt decomposes at350°C and sublimes under controlled conditions at 520 °C. Ammoniumchlorideis used as an electrolyte in dry cell batteries,as a fluxfor soldering, tinningandgalvanizing, andas a processing ingredientin textile printing and hide tanning. Use as a source of nitrogen for fertilizersis limited because of the possible build up of damaging chloride residuals in the soil.물리적 성질
Colorless cubic crystals or white granular powder; saline taste; odorless; hygroscopic; does not melt but sublimes on heating at 340°C; vapor pressure 48.75 torr at 250°C and 251.2 torr at 300°C; density 1.5274 g/cm3 at 25°C; refractive index 1.642; readily dissolves in water, solubility: 229 g and 271 g/L solution at O°C and 20°C, respectively; solubility lowered by alkali metal chlorides and HCl; dissolution lowers the temperature of the solution; sparingly soluble in alcohols (6 g/L at 19°C) and soluble in liquid NH3; insoluble in acetone and ether.출처
Ammonium chloride occurs in nature in crevices near volcanoes. Also, it is found in smoke when burning dry camel or donkey dung as fuel. Important applications of this compound include the manufacture of dry cells for batteries; as a metal cleaner in soldering; as a flux in tin coating and galvanizing; in fertilizers; in pharmaceutical applications as a diuretic, or diaphoretic expectorant; and as an analytical standard in ammonia analysis. Also, it is used in freezing mixtures; washing powders; lustering cotton; in safety explosives and in dyeing and tanning.주요 응용
Ammonium chloride is also known as sal ammoniac. white crystals made by ammonia salts acting upon hydrochloric acid followed by crystallization. It was used as a halide in many processes, including the salted paper, albumen paper, albumen opaltype, and gelatin emulsion processes. ammonium chloride is also used as a thickener and as an additive in non-alcoholic toners. According to cosmetic formulators, the ammonium component provides the tingling or stinging sensation that some people associate with toners or aftershaves, and which, in regular toners, is usually provided by the alcohol content. Ammonium chloride’s use is the result of preference in formulation feel.제조 방법
Ammonium chloride is prepared commercially by reacting ammonia with hydrochloric acid. It may be prepared by fractional crystallization from a solution containing ammonium sulphate and sodium chloride or ammonium carbonate and calcium chloride. Because of it sease of preparation it can be manufacture dindustrially along side any plant that uses or produces ammonia. Ammonium chloride is used in dry cells, metal finishing, and in the preparation of cotton for dyeing and printing.정의
ChEBI: Ammonium chloride is an inorganic chloride having ammonium as the counterion. It has a role as a ferroptosis inhibitor. It is an inorganic chloride and an ammonium salt.일반 설명
Ammonium chloride is a white crystalline solid. Ammonium chloride is soluble in water(37%). The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium chloride is used to make other ammonium compounds, as a soldering flux, as a fertilizer, and for many other uses.공기와 물의 반응
Soluble in water. Slowly releases hydrogen chloride [USCG, 1999].반응 프로필
Acidic salts, such as Ammonium chloride , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.위험도
Eye and upper respiratory tract irritant.건강위험
Inhalation of fumes irritates respiratory passages. Ingestion irritates mouth and stomach. Fumes are irritating to eyes. Contact with skin may cause irritation.Safety
Ammonium chloride is used in oral pharmaceutical formulations. The pure form of ammonium chloride is toxic by SC, IV, and IM routes, and moderately toxic by other routes. Potential symptoms of overexposure to fumes are irritation of eyes, skin, respiratory system: cough, dyspnea, and pulmonary sensitization. Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting.LD50 (mouse, IP): 1.44 g/kg
LD50 (mouse, oral): 1.3 g/kg
LD50 (rat, IM): 0.03 g/kg
LD50 (rat, oral): 1.65 g/kg
잠재적 노출
Ammonium chloride is used as an industrial chemical, pharmaceutical, and veterinary drug; to make dry batteries; in galvanizing; as a soldering flux.저장
Ammonium chloride is chemically stable. It decomposes completely at 3388℃ to form ammonia and hydrochloric acid. Store in airtight containers in a cool, dry place.운송 방법
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Purification Methods
Crystallise it several times from conductivity water (1.5mL/g) between 90o and 0o. It sublimes. After one crystallisation, ACS grade has: metal(ppm) As (1.2), K (1), Sb (7.2), V (10.2). [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 812 1963.]비 호환성
Ammonium chloride is incompatible with strong acids and strong bases. It reacts violently with ammonium nitrate and potassium chlorate, causing fire and explosion hazards. It also attacks copper and its compounds.폐기물 처리
Pretreatment involves addition of sodium hydroxide to liberate ammonia and form the soluble sodium salt. The liberated ammonia can be recovered and sold. After dilution to the permitted provisional limit, the sodium salt can be discharged into a stream or sewer.Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (oral syrup, tablets). Accepted for use as a food additive in Europe. Included in medicines licensed in the UK (eye drops; oral syrup).염화 암모늄 준비 용품 및 원자재
원자재
HEAVY CUT RESIDUE OIL
Medicinal ammonium chloride
p-클로로벤즈알데히드
Industrial grade ammonium chloride
황산 나트륨
염화나트륨
질산암모늄
이산화탄소
하이드로퀴논
Industrial ammonium chloride
염산
프로필렌 카보네이트
염화칼륨
암모늄 비카보네이트
황산암모늄
암모니아(가스)
준비 용품
3,4-디클로로-1,2,5-티아디아졸
THIOTHIAMINE
2-푸르알데히드디에틸아세탈
1-PHENYL-3-THIOSEMICARBAZIDE
4-(4-CHLOROPHENYL)-1,2,3,6-TETRAHYDROPYRIDINE HYDROCHLORIDE
2-아미노-4,6-다이메틸-3-피리딘카복사미드
엘라스타제
4-Methylbenzene-1-carboximidamide hydrochloride
황산카나마이신
Dye-fixing agent M
아미노트라이메틸렌 포스폰산
3-PYRIDINECARBOXAMIDINE
Ethyl benzoylformate
4-METHOXY-BENZAMIDINE
Methyl 3-aminosulfonylthiophene-2-carboxylate
CYCLOLEUCINOL
Praseodymium-Zircon Yellow
2-클로로-5-클로로메틸티오펜
TRIHEXYLPHOSPHINE
xylene isomerization catalysts
new type low toxic urea-formalde-hyde adhesive A-01-B
Pyridine-2-carboximidamide hydrochloride
나프틸이소시아네이트
4-AMIDINOPYRIDINIUM CHLORIDE
3-메틸티오펜-2-카르복사미드
4-Chlorobenzene-1-carboximidamide hydrochloride
4-METHYL-BENZAMIDINE
5-(AMINOMETHYL)-5-METHYLPYRROLIDIN-2-ONE
2,5-Dichlorothiophene-3-sulfonamide
2-NAPHTHYL ISOCYANATE
4-니트로벤자미딘,염화수소
2-Formylfuran-5-boronic acid
이리듐
디클로로(1,3-)-2-부텐
인터페론-감마-1a
트리사이클로헥실포스핀
4-METHOXYBENZAMIDINE, HYDROCHLORIDE
4-PYRIDINECARBOXAMIDINE
다이크로뮴산 암모늄
color fixing agent Y
염화 암모늄 공급 업체
글로벌( 1307)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei Guangda Chemical Technology Co., Ltd. | +8613607293350 |
471869703@qq.com | China | 1 | 58 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 |
aroma@qdtrustagri.com | China | 299 | 58 |
| Shijiazhuang Donghua Jinlong Chemical Co., LTD | +86-031166619888 +86-19333738986 |
donghuajinlong@glycine.com.cn | China | 25 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40068 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 |
1057@dideu.com | China | 3957 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 |
Ethan@dornechem.com | China | 3137 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |