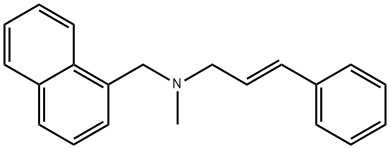
Naftifine
|
|
Naftifine 속성
- 녹는점
- 177 °C
- 끓는 점
- bp0.015 torr 162-167°
- 밀도
- 1.082±0.06 g/cm3(Predicted)
- 용해도
- Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Very Slightly)
- 물리적 상태
- 두꺼운 기름
- 산도 계수 (pKa)
- 7.99±0.50(Predicted)
- 색상
- 무색~진한 노란색
- Merck
- 6355
- CAS 데이터베이스
- 65472-88-0(CAS DataBase Reference)
안전
| HS 번호 | 29214990 |
|---|
Naftifine C화학적 특성, 용도, 생산
용도
Naftifine (Naftin) is a synthetic allylamine derivative topical antifungal agent that works by blocking squalene 2,3-epoxidase, resulting in increased cell membrane permeability and cell death. It is structurally and pharmacologically related to terbinafine. It also has some antiinflammatory properties that may be due to its ability to alter chemotaxis by polymorphonucleocytes. It is most effective against dermatophytes, moderately active against molds, and less active against yeasts, including C. albicans.Indications
Naftifine (Naftin) is a synthetic allylamine derivative topical antifungal agent that works by blocking squalene 2,3-epoxidase, resulting in increased cell membrane permeability and cell death. It is structurally and pharmacologically related to terbinafine. It also has some antiinflammatory properties that may be due to its ability to alter chemotaxis by polymorphonucleocytes. It is most effective against dermatophytes, moderately active against molds, and less active against yeasts, including C. albicans.정의
ChEBI: A tertiary amine in which the nitrogen is substituted by methyl, alpha-naphthylmethyl, and (1E)-cinnamyl groups. It is used (usually as its hydrochloride salt) for the treatment of fungal skin infections.Antimicrobial activity
Naftifine is fungicidal against dermatophytes such as Epidermophyton floccosum, Trichophyton and Microsporum species. Against pathogenic yeasts such as Candida species and moulds, it shows only an intermediate fungistatic activity in vitro.Pharmaceutical Applications
A topical antifungal used as a 1% cream for the treatment of dermatophytoses, including tinea pedis, tinea corporis and tinea cruris.Clinical Use
Naftifine was the first allyl amine to be discovered and marketed. It is subject to extensive first-pass metabolism to be orally active and, consequently, is only available in topical preparations. The widest use of naftifine is against various tinea infections of the skin.Naftifine 준비 용품 및 원자재
원자재
N-methyl-1-naphthylamine
formaldehyde
N-메틸-1-나프탈렌메틸아민 수화염화물
Para-포름알데하이드
아세토페논
Naftifine hydrochloride
아세트알데히드암모니아
탄산나트륨(경회)
Cinnamyl chloride
N,N-다이메틸폼아마이드
준비 용품
Naftifine 공급 업체
글로벌( 79)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29807 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 22866 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
sales@afinechem.com | China | 15227 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-18712993135 |
sales01@tairunfaz.com | China | 8051 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 49980 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20228 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40066 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131957 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32343 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23912 | 58 |