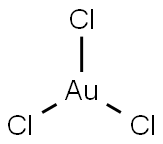
금 트리클로리드
|
|
금 트리클로리드 속성
- 녹는점
- 254°C
- 끓는 점
- 265 °C
- 밀도
- 3.9 g/mL at 25 °C
- 저장 조건
- Keep in dark place,Inert atmosphere,Room temperature
- 용해도
- DMSO(소량으로 용해), 메탄올(아주 약간 용해됨)
- 물리적 상태
- 결정질 분말, 결정 또는 덩어리
- 색상
- 노란색
- 수용성
- 녹는
- 감도
- Hygroscopic
- Merck
- 14,4521
- Solubility Product Constant (Ksp)
- pKsp: 24.5
- CAS 데이터베이스
- 13453-07-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36/37/39-45-37/39-36 | ||
| 유엔번호(UN No.) | UN 3260 8/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | MD5420000 | ||
| F 고인화성물질 | 3-8-10 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 28433000 | ||
| 기존화학 물질 | KE-18095 |
금 트리클로리드 C화학적 특성, 용도, 생산
화학적 성질
orange-red to dark red crystals물리적 성질
Red monoclinic crystals; deliquesces; density 4.7 g/cm3; sublimes at 180°C (760 torr); highly soluble in water; soluble in alcohol and ether; slightly soluble in liquid ammonia.용도
Gold(III) chloride is used in colloidal gold solutions, in photography and as a print toning agent(gold toning), starting solution to form other gold compounds and a precursor for preparation of ultra pure gold metal. It is used in electroplating and electroless plating as an anode in an electric cell. Gold(III) chloride acts as a gold catalyst and cell body stains for bright field and dark field microscopy.제조 방법
Gold(III) chloride may be produced by the combination of metallic gold with chlorine gas at elevated temperatures:2Au + 3Cl2 → 2AuCl3
It may be prepared in the laboratory by the reaction of iodine monochloride with metallic gold:
2Au + 6ICl → 2AuCl3 + 3I2
The compound should be stored tightly closed and protected from light.
정의
A compound prepared by dissolving gold in aqua regia. The bright yellow crystals (chloroauric acid) produced on evaporation are heated to form dark red crystals of gold(III) chloride. The chloride decomposes easily (at 175°C) to give gold(I) chloride and chlorine; at higher temperatures it decomposes to give gold and chlorine. Gold(III) chloride is used in photography. It exists as a dimer, Au2Cl6.화학 반응
When heated at 254ºC, gold(III) chloride decomposes to gold(I) chloride and chlorine.Passing hydrogen sulfide into an ether solution of the compound yields gold(III) sulfide, Au2S3.
A similar reaction occurs when alcoholic solutions of gold(III) chloride and hydrogen selenide are mixed, producing gold(III) selenide, Au2Se3, a black amorphous solid.
Gold(III) chloride may be reduced readily to metallic gold by common reducing agents. Thus, reduction with stannous chloride in dilute aqueous medium yields colloidal gold in which the atom carries a negative charge. “Cassius purple” is produced from the oxidation of tin to form H2Sn(OH)6, which protects colloidal gold from coagulation, imparting ruby red color to the solution.
Gold(III) chloride reacts with ammonia forming a gold(III)-nitrogen derivative, an explosive product, known as, “fulminate of gold”. Reaction with Grignard reagent, RMgX in ether yields dialkyl gold(III) chloride, R2AuCl3, which may be converted readily to other dialkyl gold(III) complexes by replacement of the chloride anion by a donor ligand.
일반 설명
The structure of gold chloride is monoclinic in nature. It sublimes at elevated temperatures.Safety Profile
Experimental reproductive effects. Human mutation data reported. Reaction with ammonia or ammonium salts yields fulminating gold, a heat-, friction-, and impact-sensitive explosive similar to mercury and silver fulminates. See also GOLD COMPOUNDS and CHLORIDES. When heated to decomposition it emits toxic fumes of Cl-.금 트리클로리드 준비 용품 및 원자재
원자재
준비 용품
금 트리클로리드 공급 업체
글로벌( 257)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 |
admin@zhanyaobio.com | China | 2136 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32836 | 60 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Nanjing Dolon Biotechnology Co.,Ltd. | 18905173768 |
sales@dolonchem.com | CHINA | 2972 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |