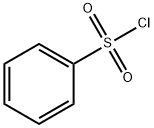
벤젠술포닐클로라이드
|
|
벤젠술포닐클로라이드 속성
- 녹는점
- 13-15 °C (lit.)
- 끓는 점
- 251-252 °C (lit.)
- 밀도
- 1.384 g/mL at 25 °C (lit.)
- 증기압
- 0.04 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.551(lit.)
- 인화점
- >230 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 알코올: 용해 가능
- 물리적 상태
- 액체
- 색상
- 투명한
- 수용성
- 분해될 수 있음
- 감도
- Moisture Sensitive
- Merck
- 14,1072
- BRN
- 606926
- 안정성
- 안정적인. 물, 강산화제, 강염기, 메틸 포름아미드, 디메틸 설폭사이드와 혼합되지 않습니다.
- LogP
- 3.39 at 23℃
- CAS 데이터베이스
- 98-09-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/22-34-42/43-29-22 | ||
| 안전지침서 | 23-26-36/37/39-45 | ||
| 유엔번호(UN No.) | UN 2225 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | DB8750000 | ||
| F 고인화성물질 | 21 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 29049090 | ||
| 유해 물질 데이터 | 98-09-9(Hazardous Substances Data) | ||
| 독성 | LD50 orl-rat: 1960 mg/kg MEPAAX 20,513,69 | ||
| 기존화학 물질 | KE-02638 |
벤젠술포닐클로라이드 C화학적 특성, 용도, 생산
화학적 성질
Benzenesulfonyl chloride is a colorless oily liquid with a pungent odor. Insoluble in water, soluble in ethanol and ether. Can react with ammonia, amine and alcohol to produce benzenesulfonamide and benzene sulfonate respectively. It is toxic, irritates skin, eyes and mucous membranes, corrosive, and can cause burns. Oral LD50 1960mg/kg in rats.용도
Benzenesulfonyl chloride reacts with Grignard reagents to form oxindoles from N-unsubstituted indoles. It is widely used to check the assay of thiamine in different food products. It is involved in the synthesis of alpha-disulfones, sulfonamides and sulfoante esters as precursor. It is a derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry. It is common reagent used in Hinsberg test for detection and distinguishing the type of amines as primary, secondary and tertiary amines.생산 방법
The most convenient method for the production of benzenesulfonyl chlorides is the chlorosulfonation reaction of benzene or substituted benzene with chlorosulfuric acid. Sulfonation and formation of the sulfonyl chlo- ride take place in one reaction sequence:Ar-H+Cl-SO3H→ArSO3H+HCl
ArSO3H+Cl-SO3H=ArSO2Cl+H2SO4
Sulfone is formed in a side reaction of the chlorosulfonation. The amount of sulfone formation can be reduced by diluting with a solvent, by using a large excess of chlorosulfuric acid, or by adding sulfone-inhibiting substances, e.g., alkali metal and ammonium salts, acetic acid, phosphoric acid, dimethylformamide, or amidosulfuric acid.
For industrial chlorosulfonation the chlorosulfuric acid is introduced into a cast-steel or enameled steel vessel and 10 – 25 mol% of the aromatic compound is stirred in at 25 – 30 ℃, whereupon sulfonation of the aromatic compound and HCl formation occur. The formation of sulfonyl chloride is initiated by heating the reactants to 50 – 80 ℃. The reaction is exothermic. The sulfonyl chloride is isolated by draining the reaction mass onto water and simultaneous cooling. Excess chlorosulfuric acid is decomposed, and the sulfonyl chloride either precipitates or separates as an organic liquid phase. The quality of the chlorosulfuric acid affects the yield.
Other chlorinating agents, such as phosgene, thionyl chloride, sulfuryl chloride, or phosphorus pentachloride, can be used instead of chlorosulfu- ricacid. When thionyl chloride is used the sulfonyl chloride is obtained from the sulfonic acid in high yield and without formation of sulfuric acid.
일반 설명
A colorless to slightly yellow solid that melts at approximately 40°F. Very irritating to skin, eyes and mucous membranes. May emit toxic fumes when heated to high temperatures. Used to make dyes and other chemicals.공기와 물의 반응
Insoluble and stable in cold water [Merck]. Decomposes in hot water to produce corrosive and toxic hydrochloric acid and benzenesulfonic acid. Rate of reaction decreases as temperature decreases.반응 프로필
Benzenesulfonyl chloride is incompatible with strong oxidizing agents and bases, including amines. Corrodes metals in the presence of water due to slow formation of hydrochloric acid and benzenesulfonic acid [USCG, 1999]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].건강위험
May be fatal if inhaled, swallowed or absorbed through skin. Contact may cause skin and eye burns. Irritating to eyes, skin and mucous membranes. INGESTION: May cause abdominal spasm and vomiting.Safety Profile
Poison by intraperitoneal route. Adangerous storage hazard. It may explode in a sealedbottle. Explosive reaction with dimethyl sulfoxide. Reactsvigorously with methyl formamide. When heated todecomposition it emits toxic fumes of Cl- and SO잠재적 노출
It is used as a chemical intermediate for benzenesulfonamides, thiophenol, glybuzole (hypoglycemic agent), N-2-chloroehtylamides, benzonitrile; for its esters-useful as insecticides, and miticides.운송 방법
UN2225 Benzene sulfonyl chloride, Hazard class: 8; Labels: 8—Corrosive material.Purification Methods
Distil the sulfonyl chloride, then treat it with 3mole % each of toluene and AlCl3, and allow it to stand overnight. The sulfonyl chloride is distilled off at 1mm pressure and then carefully fractionally distilled at 10mm in an all-glass column. [Adams & Marvel Org Synth Coll Vol I 84 1941, Jensen & Brown J Am Chem Soc 80 4042 1958, Beilstein 11 IV 49.] It is TOXIC.비 호환성
Violent reaction with strong oxidizers, dimethyl sulfoxide, and methyl formamide. It is very reactive with bases and many organic compounds. Incompatible with ammonia, aliphatic amines. Water contact forms hydrochloric and chlorosulfonic acids. Aqueous solutions of this chemical are strong acids that react violently with bases. Attacks metals in presence of moisture.벤젠술포닐클로라이드 준비 용품 및 원자재
원자재
준비 용품
2-(2,3-DIHYDRO-1BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ETHANAMINE
설파살라진
Azoic Diazo Component 34
(트리 플루오로 메틸) 트리메틸 실란
17-hydroxypregna-4,9(11)-diene-3,20-dione
2-(2-CHLOROETHOXY)-BENZENESULFONAMIDE
다이벤젠설폰이미드
설파퀴녹살린
티오페놀
16alpha,17-epoxypregna-4,9(11)-diene-3,20-dione
벤젠설폰아미드
2-Methyl-3-nitroaniline
벤젠설포닉 산, 하이드라자이드
BENZENETHIONOSULFONIC ACID, SODIUM SALT
에디펜포스
2,4-디클로로-5-설파모일벤조익 산
dimethyl p-octadecyl phenylsulfonyl amino propyl ammoium propylsulfonate
파라-톨루엔설폰일 염화물
Acid Yellow 79
2-메틸-4-나이트로아닐린
2-(2,3-DIHYDRO-1-BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ACETONITRILE
카르브아졸 디옥사진 바이올렛
Leuprorelin
Benzenesulfonylazide
1-(PHENYLSULFONYL)-1H-INDOLE-2-CARBALDEHYDE
벤젠술폰산나트륨
4-메틸-2-니트로아닐린
2,5-Dichloropyridine
벤젠술포닐클로라이드 공급 업체
글로벌( 402)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |