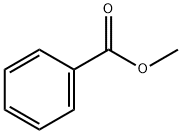
안식향산메틸
|
|
안식향산메틸 속성
- 녹는점
- -12 °C (lit.)
- 끓는 점
- 198-199 °C (lit.)
- 밀도
- 1.088 g/mL at 20 °C (lit.)
- 증기 밀도
- 4.68 (vs air)
- 증기압
- <1 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.516(lit.)
- 인화점
- 181 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 에탄올: 용해성60%, 투명(1mL/4ml)
- 물리적 상태
- 액체
- Specific Gravity
- 1.087~1.095 (20℃)
- 색상
- 무색~담황색 투명
- 냄새
- 디프로필렌 글리콜 중 1.00%. 페놀릭 윈터그린 아몬드 플로럴 카낭가
- ?? ??
- 페놀성
- 폭발한계
- 8.6-20%(V)
- 수용성
- 22.5&C에서 <0.1g/100mL
- JECFA Number
- 851
- Merck
- 14,6024
- BRN
- 1072099
- Dielectric constant
- 6.6(20℃)
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 강산, 강염기와 호환되지 않습니다.
- LogP
- 2.12-2.2 at 20℃
- CAS 데이터베이스
- 93-58-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22 | ||
| 안전지침서 | 36 | ||
| 유엔번호(UN No.) | UN 2938 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | DH3850000 | ||
| 자연 발화 온도 | 510 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29163100 | ||
| 유해 물질 데이터 | 93-58-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 3.43 g/kg (Smyth) | ||
| 기존화학 물질 | KE-23483 |
안식향산메틸 C화학적 특성, 용도, 생산
개요
Methyl benzoate is an organic compound. It is an ester with the chemical formula C6H5CO2CH3. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.화학적 성질
Methyl Benzoate has been found in essential oils (e.g., ylang-ylang oil). It is a colorless liquid with a strong, dry-fruity, slightly phenolic odor. Methyl benzoate can be converted simply into other benzoates by transesterification. Since methyl benzoate is a fairly large by-product in the manufacture of Terylene, earlier synthetic routes such as those starting from benzoic acid or benzoyl chloride have largely been abandoned.물리적 성질
Methyl benzoate is a colorless, oily, transparent, liquid that has a pleasant fruity odor, similar to cananga. miscible with ether, soluble in methanol and ether, insoluble in water and glycerol. It is used in perfume bases, such as ylang-ylang and tuberose types.출처
Methyl benzoate can be isolated from the freshwater fern Salvinia molesta. It is one of many compounds that is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.Cocaine hydrochloride hydrolyzes in moist air to give methyl benzoate; drug - sniffing dogs are thus trained to detect the smell of methyl benzoate.
정의
ChEBI: Methyl benzoate is a benzoate ester obtained by condensation of benzoic acid and methanol. It has a role as a metabolite and an insect attractant. It is a benzoate ester and a methyl ester.제조 방법
Methyl benzoate is manufactured by heating benzoic acid and dimethyl sulfate to high temperature, or by exchange between ethyl benzoate and methanol in KOH solution. It may also be produced by the alcoholysis of benzonitrile. It is a by-product of ozonolysis of water.주요 응용
Methyl benzoate is a volatile aromatic ester compound widely used in perfumery industries. It is naturally occurring in guava, mango, and kiwifruit. It is one of many compounds that is attractive to males of various species of orchid bees, who apparently gather the chemical to synthesize pheromones. It can also be utilized as a precursor for:Selective synthesis of benzaldehyde using supported manganese oxide catalysts.
Preparation of benzophenone derivatives by reacting with aryl compounds via Friedel-Crafts acylation.
일반 설명
A crystalline solid or a solid dissolved in a liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.공기와 물의 반응
Slightly soluble in water. Hydrolyzes slowly in contact with water .반응 프로필
Methyl benzoate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Methyl benzoate reacts with strong oxidizing agents and strong bases and hydrolyzes slowly in contact with water. .위험도
Toxic by ingestion.건강위험
Methyl benzoate is a mild skin irritant. Irritating to the eyes, nose, throat, upper respiratory tract, and skin. May cause allegic skin and respiratory reactions.Theacute oral toxicity in test animals was oflow order. The toxic symptoms in animalsfrom oral administration of this compoundwere tremor, excitement, and somnolence.The LD50 value varies with species. The oralLD50 values in mice and rats are 3330 and1350 mg/kg, respectively.Safety Profile
Moderately toxic by ingestion. Mildly toxic by skin contact. A skin and eye irritant. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical, water to blanket fire. When heated to decomposition it emits acrid smoke and irritating fumes.잠재적 노출
Used as food additive and as a solvent for cellulose esters and ethers, resins and rubber.Purification Methods
Wash the ester with dilute aqueous NaHCO3, then water, dry with Na2SO4 and fractionally distil it in a vacuum. [Beilstein 9 IV 283.]폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.안식향산메틸 준비 용품 및 원자재
원자재
수소
메틸알콜
벤조산
Methyl 4-iodobenzoate
N'-benzoyl-N,N-diethylurea
oxanthranol
1-METHYL-4-PHENYLETHYNYL-BENZENE
준비 용품
안스라닐산메틸
벤조산노르말프로필에스테르
3-Nitrobenzoic acid
Lactofen
3-AMINO-1-PHENYL-PROPAN-1-OL
Levosulpiride
Benzohydroxamic acid
2-Ethoxybenzoic acid
Methyl 3,4,5-trimethoxybenzoate
Methyl 3-nitrobenzoate
Methyl 2-bromobenzoate
1,3,2-Dioxaborolane, 4,4,5,5-tetramethyl-2-(phenylmethoxy)-
2-P-ANISYL-1,3-INDANDIONE
benzoylazide
안식향산메틸 공급 업체
글로벌( 526)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Wuhan Biet Co., Ltd. | +8617320528784 |
min@biet.com.cn | China | 41 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| China Synchem Technology Co.,Ltd. | +86-0552-4929304 +86-18055277008 |
wangxuhui1985@126.com | China | 55 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 |
stella@zetchem.com | China | 2141 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |