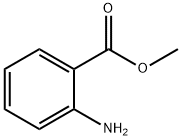
안스라닐산메틸
|
|
안스라닐산메틸 속성
- 녹는점
- 24 °C (lit.)
- 끓는 점
- 256 °C (lit.)
- 밀도
- 1.168 g/mL at 25 °C (lit.)
- 증기압
- 1 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.582(lit.)
- 인화점
- 220 °F
- 저장 조건
- Keep in dark place,Inert atmosphere,Room temperature
- 용해도
- 알코올: 자유롭게 용해됨(lit.)
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- pK1:2.23(+1) (25°C)
- 색상
- 맑은 황갈색
- 냄새
- 포도 냄새
- 수소이온지수(pH)
- 7.5-8 (H2O, 20℃)Aqueous solution
- 폭발한계
- 1.4-7.8%(V)
- ?? ??
- 과일 같은
- 수용성
- 약간 용해됨
- 감도
- Air Sensitive
- Merck
- 14,6020
- JECFA Number
- 1534
- BRN
- 606965
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제와 호환되지 않습니다.
- InChIKey
- VAMXMNNIEUEQDV-UHFFFAOYSA-N
- LogP
- 1.88 at 20℃
- CAS 데이터베이스
- 134-20-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-37/39 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | CB3325000 | ||
| F 고인화성물질 | 21 | ||
| 자연 발화 온도 | 986 °F | ||
| TSCA | Yes | ||
| HS 번호 | 29224995 | ||
| 유해 물질 데이터 | 134-20-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats, mice: 2910, 3900 mg/kg, P. M. Jenner et al., Food Cosmet. Toxicol. 2, 327 (1964) | ||
| 기존화학 물질 | KE-01204 |
안스라닐산메틸 C화학적 특성, 용도, 생산
순도시험
(1) 응고점 : 이 품목의 응고점은 23.8℃이상이어야 한다.
(2) 용상 : 이 품목 1mL를 30℃로 가온하여 녹인 다음 60% 에탄올 6mL에 녹일 때, 그 액은 징명하여야 한다.
(3) 굴절률 : 이 품목의 굴절률 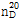 은 1.582~1.584이어야 한다.
은 1.582~1.584이어야 한다.
(4) 산가 : 이 품목의 산가는 향료시험법 중 산가측정법에 따라 시험할 때, 1 이하이어야 한다.
확인시험
(1) 이 품목 0.1g을 염산(1→10)에 녹이고 아질산나트륨시액 1mL 및 β-나프톨 0.1g을 수산화나트륨시액 5mL에 녹인 액 2mL를 넣으면 등적색의 침전이 생긴다.
(2) 이 품목 1g에 10% 알콜성수산화칼륨용액 5mL를 넣고 수욕 중에서 가열한 다음 물 5mL를 섞어 식힌 다음 묽은염산 4mL를 넣으면 백~회색의 침전이 생긴다.
정량법
이 품목 0.5g을 정밀히 달아 향료시험법 중 에스테르가 및 에스테르함량측정법에 따라 시험한다.
0.5N 알콜성수산화칼륨용액 1mL = 75.58mg C8H9NO2
개요
Methyl anthranilate, also known as MA, methyl 2-amino benzoate or carbo methoxy aniline, is an ester of anthranilic acid. Its chemical formula is C8H9NO2.화학적 성질
Methyl Anthranilate occurs in a large number of blossom essential oils (e.g., neroli, ylang-ylang, and jasmine oils), grapes, and citrus oils. It occurs as white crystals (mp 24–25°C), or a yellowish liquid, that show blue fluorescence and have an orange blossom odor. Methyl anthranilate is prepared by esterification of anthranilic acid with methanol or by reaction of isatoic anhydride with methanol.It is used in a large number of blossom fragrances. However, its use in perfumes for soaps and cosmetics is limited because it causes discoloration. It is used in flavor compositions (e.g., in grape and citrus flavors).
출처
Methyl anthranilate naturally occurs in the Concord grapes and other Vitis labrusca grapes or hybrids thereof, and in bergamot, black locust, champaca , gardenia, jasmine, lemon, mandarin, neroli, oranges, rue oil, strawberry, tuberose, wisteria, galangal and ylang ylang. It is also a primary component of the essential apple flavor, along with ethyl acetate and ethyl butyrate.It is also secreted by the musk glands of foxes and dogs, and lends a "sickly sweetness" to the smell of rotting flesh.용도
Methyl anthranilate acts as a bird repellent. It is food-grade and can be used to protect corn, sunflowers, rice, fruit, and golf courses. Dimethyl anthranilate (DMA) has a similar effect. It is also used for the flavor of grape Kool Aid. It is used for flavoring of candy, soft drinks (e.g. grape soda), gums, and drugs.Methyl anthranilate both as a component of various natural essential oils and as a synthesised aroma-chemical is used extensively in modern perfumery . It is also used to produce Schiff's Bases with aldehydes, many of which are also used in perfumery. In a perfumery context the most common Schiff's Base is known as aurantiol - produced by combining methyl anthranilate and hydroxyl citronellal.
정의
ChEBI: Methyl anthranilate is a benzoate ester that is the methyl ester of anthranilic acid. It has a role as a metabolite and a flavouring agent. It derives from an anthranilic acid.제조 방법
By heating anthranilic acid and methyl alcohol in the presence of sulfuric acid and subsequent distillation.일반 설명
Clear colorless to tan liquid with an odor of grapes. Has light blue fluorescence.공기와 물의 반응
Methyl anthranilate is sensitive to air and light. Slightly water soluble .반응 프로필
An amine and ester. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.화재위험
Methyl anthranilate is combustible.Toxicology
Methyl anthranilate is a colorless liquid that has a sweet, fruity, grape-like flavor. It is found in the essential oils of orange, lemon, and jasmine and has been widely used to create imitation Concord grape flavor. Table 10.8 shows the acute toxicity of methyl anthranilate. Methyl anthranilate promotes some allergic reactions on human skin, which has led to it being prohibited for use in cosmetic products.Safety Profile
Moderately toxic by ingestion. Experimental reproductive effects. A skin irritant. See also ESTERS. Combustible liquid. When heated to decomposition it emits toxic fumes of NOx.Safety
Methyl anthranilate is a plant-based compound with a long history of use as a flavor additive for foods and beverages, and as an aromatic used extensively in perfumery. As such, the US Department of Agriculture (USDA) and the Food and Drug Administration (FDA) have approved MA as "generally recognized as safe".신진 대사
It is probable that this ester is hydrolysed and the anthranilate is excreted mostly as oaminobenzoyl glucuronide (Charconnet-Harding, Dalgliesh & Neuberger, 1953).Solubility in organics
Methyl anthranilate is soluble in ethanol and propylene glycol. It is insoluble in paraffin oil.안스라닐산메틸 준비 용품 및 원자재
원자재
염화 제일석
벤조 산, 나트륨 염
Methanol solution
차아염소산나트륨
요오드화 칼륨
커먼자스민오일
땅콩 기름
벤즈아마이드
aminoformic acid
포름산나트륨
소다의 염산
안식향산메틸
오렌지꽃오일
안트라닐산
니트로벤조산
AminobenzoicAcid
준비 용품
2-AMINO-3,5-DIBROMOBENZYL ALCOHOL
Methyl 2-amino-3,5-dibromobenzoate
사카린 나트륨, 이수화물
사카린
메틸 N-메틸안트라닐레이트
1,2-benzisothiazol-3(2H)-one 1,1-dioxide, ammonium salt
Pigment Red 175
Bromhexine hydrochloride
아진포스 에틸
SACCHARIN SODIUM SALT DIHYDRATE
벤타존
Potassium saccharate
Ambroxol
칼슘사카린
Methyl 2-(chlorosulfonyl)benzoate
METHYL BIPHENYL-2-CARBOXYLATE
2,2'-이미노디벤조산
안스라닐산메틸 공급 업체
글로벌( 637)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Changzhou AniKare Pharmatech Co., Ltd. | +86-0519-8359-8696 +8618018249389 |
sales@anikare.com | China | 8382 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Hunan aslsen technology co.,ltd | +8619313031929 |
aslsd@aslsen.com | China | 128 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 951 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 2259 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |