- Losartan
-

-
2025-08-10
- CAS:114798-26-4
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Losartan
-

- $0.00 / 25KG
-
2025-08-08
- CAS:114798-26-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Losartan
-

- $238.00 / 1Kg/Bag
-
2025-07-29
- CAS:114798-26-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1T
|
| | Losartan Basic information |
| Product Name: | Losartan | | Synonyms: | 2-Butyl-4-chloro-5-(hydroxymethyl)-1-[(2'-(1H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole;Compound 89;2-n-butyl-4-chloro-5-hydroxyMethyl-1-[(2'-(1H-tetrazole-5-yl)biph;2-Butyl-4-chloro-1-[[2'-(2H-tetrazol-5-yl)-biphenyl-4-yl]methyl]-1H-imidazol-5-methanol;(2-butyl-4-chloro-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazol-5-yl)methanol;dup89;nyl)-4-yl)methyl)-;2-butyl-4-chloro-1-[p-(o-1h-tetrazol-5-ylphenyl)benzyl]imidazole-5- methanol | | CAS: | 114798-26-4 | | MF: | C22H23ClN6O | | MW: | 422.91 | | EINECS: | 601-329-8 | | Product Categories: | Isotopically Labeled Pharmaceutical Reference Standard | | Mol File: | 114798-26-4.mol | 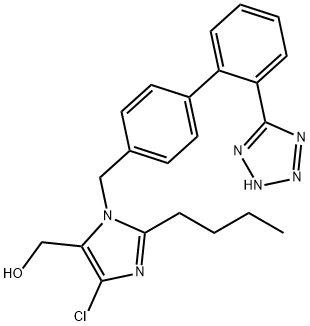 |
| | Losartan Chemical Properties |
| | Losartan Usage And Synthesis |
| Originator | Alsartan, Aristo Pharmaceutical Ltd., India | | Uses | Losartan is a nonpeptide angiotensin II AT1-receptor antagonist. Antihypertensive. | | Uses | antihypertensive;antagonist of angiotensin type 1 | | Definition | ChEBI: A biphenylyltetrazole where a 1,1'-biphenyl group is attached at the 5-position and has an additional trisubstituted imidazol-1-ylmethyl group at the 4'-position | | Manufacturing Process | 2-Butyl-4-chloro-1-(2'-(tetrazol-5-yl)biphenyl-4-ylmethyl)-1H-imidazole-5- methanolpotassium was synthesized in 5 stages.
1. Methyl 4'-methylbiphenyl-2-carboxylate (44.2 mmol), 0.5 N KOH in methanol (133 mmol), and water (50 mL) were mixed and refluxed under nitrogen. After 5 hours, the solvent was removed in vacuo and water (200 mL) and ethyl acetate (200 mL) added. The aqueous layer was acidified with concentrated hydrochloric acid to a pH of 3 and the layers were separated. The aqueous phase was extracted with ethyl acetate, the organic layers collected, dried (MgSO4) and the solvent removed in vacuo to yield 8.71 g of a 4'-methylbiphenyl-2-carboxylic acid, melting point 140.0-145.0°C.
2. 4'-Methylbiphenyl-2-carboxylic acid (41 mmol) and thionyl chloride (411 mmol) were mixed and refluxed for 2 hours. The excess thionyl chloride was removed in vacuo and the residue was taken up in toluene. The toluene was removed by rotary evaporation. The crude acid chloride was then added slowly to cold (0°C) concentrated NH4OH (50 mL) so that the temperature was kept below 15°C. After 15 minutes of stirring, water (100 mL) was added and solids precipitated. These were collected, washed with water and dried under high vacuum over P2O5 to yield 7.45 g of a white solid, melting point 126.0-128.5°C. The above product amide (35 mmol) and thionyl chloride (353 mmol) were mixed and refluxed for 3 hours. The thionyl chloride was removed using the same procedure as described above. The residue was washed with a little hexane to yield 6.64 g of 4'-methyl-2-cyanobiphenyl, melting point 44.0- 47.0°C.
3. 4'-Methyl-2-cyanobiphenyl (5.59 g) was brominated using benzoyl peroxide as an initiator. The product was recrystallized from ether to yield 4.7 g of 4'- bromomethyl-2-cyanobiphenyl, melting point 114.5-120.0°C.
4. 4'-Bromomethyl-2-cyanobiphenyl (4.6 g) was alkylated onto 2-n-butyl-4-
chloro-5-(hydroxymethyl)-imidazole. For separation of the product was used a
flash chromatography in 1:1 hexane/ethyl acetate over silica gel. The
regioisomeric products yielded 2.53 g of the faster eluting isomer.
Recrystallization from acetonitrile yielded 1.57 g of analytically pure 2-n-butyl4-chloro-1-[2'-cyanobiphenyl-4-yl)methyl]-5-(hydroxymethyl)-imidazole,
melting point 153.5 -155.5°C.
5. 2-n-Butyl-4-chloro-1-[(2'-cyanobiphenyl-4-yl)-methyl]-5-(hydroxymethyl)-
imidazole (10 mmole), sodium azide (10 mmol), and ammonium chloride (30
mmol) were mixed in DMF (150 mL) under N2 at 100°C for 2 days, after
which the temperature was raised to 120°C for 6 days. The reaction was
cooled and 3 more equivalents each of ammonium chloride and sodium azide
were added. The reaction was again heated for 5 days at 120°C. The reaction
was cooled, the inorganic salts filtered, and the filtrate solvent removed in
vacuo. Water (200 mL) and ethyl acetate (200 mL) were added to the residue
and the layers were separated. The aqueous layer was extracted with ethyl
acetate, the organic layers were collected, dried (MgSO4) and the solvent
removed in vacuo, to yield a dark yellow oil. The product was purified by flash
chromatography in 100% ethyl acetate to 100% ethanol over silica gel to
yield 5.60 g of a light yellow 2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1Htetrazol-5-yl)biphenyl-4-yl)methyl]imidazole. Recrystallization from acetonitrile
yielded 4.36 g of light yellow crystals which still melted broadly. The crystals
were taken up in 100 mL of hot acetonitrile. The solid that did not dissolve
was filtered off to yield 1.04 g of product as a light yellow solid, melting point
of 2-n-butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-
yl)methyl]imidazole 183.5-184.5°C.
2-n-Butyl-4-chloro-5-hydroxymethyl-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-
yl)methyl]imidazole may be converted to potassium salt. | | Brand name | Cozaar (Merck). | | Therapeutic Function | Antihypertensive | | General Description | Losartan, 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-yl-phenyl)benzyl]imidazole-5-methanol monopotassiumsalt (Cozarr), was the first nonpeptide imidazole to beintroduced as an orally active angiotensin II antagonist withhigh specificity for AT1. When administered to patients, itundergoes extensive first-pass metabolism, with the 5-methanol being oxidized to a carboxylic acid. This metabolismis mediated by CYP 2C9 and 3A4 isozymes. The 5-methanol metabolite is approximately 15 times more potentthan the parent hydroxyl compound. Because the parent hydroxylcompound has affinity for the AT1 receptor, strictlyspeaking, it is not a prodrug. | | Flammability and Explosibility | Non flammable | | Biological Activity | Losartan is an orally available, potent, and selective competitive angiotensin II receptor type 1 (AT1) antagonist. Losartan is used for treatment of hypertension. |
| | Losartan Preparation Products And Raw materials |
|