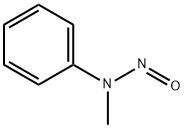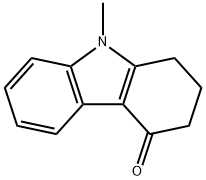
1,2,3,4-Tetrahydro-9-methylcarbazol-4-one synthesis
- Product Name:1,2,3,4-Tetrahydro-9-methylcarbazol-4-one
- CAS Number:27387-31-1
- Molecular formula:C13H13NO
- Molecular Weight:199.25
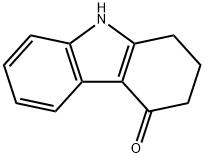
15128-52-6
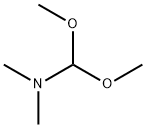
4637-24-5

27387-31-1
The reaction was carried out in N,N-dimethylformamide (5.0 mL) at reflux for 1.0 h with 2,3-dihydro-1H-carbazol-4(9H)-one (1.0 mmol) and N,N-dimethylformamide dimethyl acetal (10 mmol). After completion of the reaction, the mixture was cooled to room temperature and extracted with ethyl acetate (2 x 25 mL). The organic layer was separated and washed with saturated brine solution and subsequently dried over anhydrous Na2SO4. The solvent was removed by concentration under reduced pressure and the resulting solid was recrystallized from ethanol to give white acicular crystals in 71% yield, melting point 188-190 °C. IR (cm-1 ): 1695 (C=O). 1H NMR (400 MHz, CDCl3) δ: 2.24-2.27 (t, 2H, CH2, J = 6.28 Hz), 2.56-2.59 (t, 2H, CH2, J = 6.0 Hz), 2.56-2.59 (t, 2H, CH2, J = 6.0 Hz). CH2, J = 6.0 Hz), 2.91-2.94 (t, 2H, CH2, J = 6.21 Hz), 3.70 (s, 3H, NCH3), 7.27-7.30 (m, 3H, Ph), 8.24-8.26 (m, 1H, Ph). Calculated elemental analysis (C13H13NO): C, 78.36; H, 6.58; N, 7.03%. Measured values: C, 78.29; H, 6.49; N, 6.92%.

15128-52-6
289 suppliers
$6.00/5g

4637-24-5
646 suppliers
$5.00/25g

27387-31-1
268 suppliers
$8.00/5g
Yield:27387-31-1 71%
Reaction Conditions:
in N,N-dimethyl-formamide; for 1 h;Reflux;
Steps:
9-Methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one (1)
A mixture of 2,3-Dihydro-1H-carbazol-4(9H)-one (1.0 mmol)and DMF-DMA (10 mmol) in DMF (5.0 mL) was refluxed for 1.0 h.The reaction mixture was cooled and extracted with ethyl acetate(2 25 mL). The organic layer was separated and washed with brine solution. The organic layer was dried over anhydrous Na2SO4.The solvent was removed under reduced pressure and the solidobtained was recrystallized from ethanol.White needles, yield 71%, m.p. 188-90 C. IR (cm1) 1695(CO), 1H NMR (400 MHz, CDCl3) d 2.24-2.27 (t, 2H, CH2,J = 6.28 Hz), 2.56-2.59 (t, 2H, CH2, J = 6.0 Hz), 2.91-2.94 (t, 2H,CH2, J = 6.21 Hz), 3.70 (s, 3H, NCH3), 7.27-7.30 (m, 3H, Ph), 8.24-8.26 (m, 1H, Ph). Anal. Calc. for C13H13NO: C, 78.36; H, 6.58, N,7.03%. Found: C, 78.29; H, 6.49; N, 6.92%.
References:
Gautam, Deepika;Chaudhary [Journal of Molecular Structure,2015,vol. 1080,p. 137 - 144]
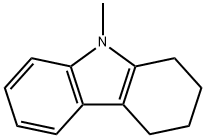
6303-88-4
8 suppliers
inquiry

27387-31-1
268 suppliers
$8.00/5g

15128-52-6
289 suppliers
$6.00/5g

77-78-1
319 suppliers
$22.00/25g

27387-31-1
268 suppliers
$8.00/5g
57466-25-8
0 suppliers
inquiry

27387-31-1
268 suppliers
$8.00/5g