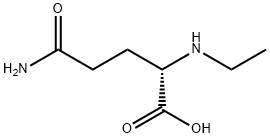
L-Theanine
- CAS No.
- 3081-61-6
- Chemical Name:
- L-Theanine
- Synonyms
- theanine;N5-Ethyl-L-glutamine;Theanin;SUNTHEANINE;N-Ethyl-L-glutamine;L-Theanine 3081-61-6;THEA;NSC 21308;-Theanine;L-TheaMine
- CBNumber:
- CB1485976
- Molecular Formula:
- C7H14N2O3
- Molecular Weight:
- 174.2
- MDL Number:
- MFCD00059653
- MOL File:
- 3081-61-6.mol
| Melting point | 207°C |
|---|---|
| Boiling point | 430.2±40.0 °C(Predicted) |
| Density | 1.171±0.06 g/cm3(Predicted) |
| refractive index | 8 ° (C=5, H2O) |
| storage temp. | 2-8°C |
| solubility | Soluble in Water (up to 20 mg/ml). |
| pka | 2.24±0.10(Predicted) |
| form | powder |
| color | White |
| Odor | Odorless |
| Water Solubility | almost transparency |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 2 months. |
| InChI | InChI=1S/C7H14N2O3/c1-2-9-5(7(11)12)3-4-6(8)10/h5,9H,2-4H2,1H3,(H2,8,10)(H,11,12)/t5-/m0/s1 |
| InChIKey | DATAGRPVKZEWHA-YFKPBYRVSA-N |
| SMILES | C(O)(=O)[C@H](CCC(N)=O)NCC |
| LogP | -0.661 (est) |
| CAS DataBase Reference | 3081-61-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8021PR16QO |
L-Theanine price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SMB00395 | L-Theanine ≥98% (HPLC) | 3081-61-6 | 100mg | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHL82213 | L-Theanine phyproof? Reference Substance | 3081-61-6 | 20MG | $288 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1652704 | L-Theanine United States Pharmacopeia (USP) Reference Standard | 3081-61-6 | 200mg | $420 | 2024-03-01 | Buy |
| TCI Chemical | T0954 | L-Theanine >98.0%(T) | 3081-61-6 | 1g | $89 | 2024-03-01 | Buy |
| TCI Chemical | T0954 | L-Theanine >98.0%(T) | 3081-61-6 | 5g | $301 | 2024-03-01 | Buy |
L-Theanine Chemical Properties,Uses,Production
Physiochemical properties
L-Theanine(3081-61-6), like the protein-based amino acids, exists as a zwitterionic species and is a colorless crystalline solid [needles, melting point 214-216 ℃][2]. Studies on the buffering capacity of green tea extracts suggest the pKa of the theanine amino group to be[10, 12]. The pKa of the carboxyl unit was not formally quantified due to interference from other acidic species. However, comparisons with close structural analogues such as glutamine suggest the value lies in the range 2.1-2.5[13] Theanine is stable under acidic conditions but undergoes base hydrolysis to yield glutamic acid and ethylamine[2, 14]. During infusion, theanine does not react chemically with any of the other tea components. This is in contrast to catechins, which can precipitate from solution as a result of π stacking interactions with caffeine[15, 16]or can react with proteins and enzymes such as lipoxygenase, α-amylase, pepsin, trypsin and lipase[15].
Health effects
Green tea leaves have many beneficial health activities including anti-inflammatory, anti-carcinogenic, anti-mutagenic, antioxidative and antimicrobial and hypolipidemic effects[38, 39]. Those beneficial effects are largely originated from the biological effects from L-Theanine as below:
Ingestion of theanine has been reported to facilitate the generation of alpha brain waves, which are associated with a relaxed but alert mental state[40]. In addition, theanine is reported to promote the release of the inhibitory neurotransmitter γ-aminobutyric acid [GABA], which in turn regulates dopamine and serotonin levels in the brain[41]. Thus, theanine consumption has been closely associated with relaxation and improved learning ability.
Safety information
FDA classified L-theanine as “generally recognized as safe.” This classification means that they believe this additive to be safe when people use it as the packaging suggests.
Women who are pregnant or breastfeeding should not use L-theanine.
Reference
1 Sakato Y, The chemical constituents of tea: III. A new amide theanine. Nippon Nogei Kagakukaishi 23:262–267 [1949].
2 Wan X, Zhang Z and Li D, Chemistry and biological properties of theanine, in Tea and Tea Products, ed.byHoCT, Lin JKandShahidi F. CRC Press, Boca Raton, pp. 255–274 [2009].
3. G. Eschenauer, B.V. Sweet, Pharmacology and therapeutic uses of theanine, Am. J. Health Syst. Pharm. 63 [1][2006]28–30.
4. D.J. White, S. de Klerk, W. Woods, S. Gondalia, C. Noonan, A.B. Scholey, Antistress, behavioural and magnetoencephalography effects of an L-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial, Nutrients 8 [1][2016]53.
5. M.S. Butt, R.S. Ahmad, M.T. Sultan, M.M.N. Qayyum, A. Naz, Green tea and anticancer perspectives: updates from last decade, Crit. Rev. Food Sci. Nutr. 55 [6][2015]792–805.
6. V. Crespy, G. Williamson, A review of the health effects of green tea catechins in in vivo animal models, J. Nutr. 134 [12][2004]3431–3440.
7. R. Cooper, Green tea and theanine: health benefits, Int. J. Food Sci. Nutr. 6 [3][2012]90–97.
8. Juneja LR, ChuDC, Okubo T,Nagato Y and Yokogoshi H, L-Theanine – a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Technol 10:199–204 [1999].
9. Balentine DA, Harbowy ME and Graham HN, Tea: The plant and its manufacture; chemistry and consumption of the beverage, in Caffeine, ed. by Spiller GA. CRC Press, Boca Raton, pp. 35–72 [1998].
10. Chu DC, Green tea – its cultivation, processing of the leaves for drinking materials, and kinds of green tea, in Chemistry and Applications of Green Tea, ed. by Yamamoto T, Juneja LR, Chu DC and Kim M. CRC Press, Boca Raton, pp. 1–11 [1997].
11. Deng WW, Ogita S and Ashihara H, Biosynthesis of theanine [γ ethylamino-l-glutamic acid]in seedlings of Camellia sinensis. Phytochem Lett 1:115–119 [2008].
12. Chu DC, Kobayashi K, Juneja LR and Yamamoto T, Theanine – its synthesis, isolation, and physiological activity, in Chemistry and Applications of Green Tea, ed. by Yamamoto T, Juneja LR, Chu DC and Kim M. CRC Press, Boca Raton, pp. 129–135 [1997].
13. Yamano HandMiyagawa K,Buffer capacity curves of green tea extracts using a personal computer with numerically treated online software. Food Sci Technol Int Tokyo 3:69–73 [2009].
14. Ekborg-Ott KH, Taylor A and Armstrong DW, Varietal differences in the total and enantiomeric composition of theanine in tea. J Agric Food Chem 45:353–363 [1997].
15. Vuong VQ, Golding JB, Nguyen M and Roach PD, Extraction and isolation of catechins from tea. J Sep Sci 33:3415–3428 [2010].
16. Ishizu T, Tsutsumi H and Sato T, Interaction between gallocatechin gallate and caffeine in crystal structure of 1 : 2 and 2 : 2 complexes. Tetrahedron Lett 50:4121–4124 [2009].
17. Liang H, Liang Y, Dong J, Lu J, Xu H and Wang H, Decaffeination of fresh green tea leaf [Camellia sinensis]byhotwater treatment. Food Chem 101:1451–1456 [2007].
18 Narukawa M, Morita K and Hayshi Y, L-Theanine elicits an umami taste with inosine 5’-monophosphate. Biosci Biotechnol Biochem 72:3015–3017 [2008].
19 de Araujo IET, Kringelbach ML, Rolls ET and Hobden P, Representation of umami taste in the human brain. J Neurophysiol 90:313–319 [2003].
20 Y. Kim, K.L. Goodnera, J.D. Parkb, J. Choib, S.T. Talcott, Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation, Food Chem. 129 [4][2011]1331–1342.
21 W.W. Deng, S. Ogita, H. Ashihara, Biosynthesis of theanine [γ-ethylamino-l-glutamic acid]in seedlings of Camellia sinensis, Phytochem. Lett. 1 [2][2008]115–159.
22 M. Narukawa, Y. Toda, T. Nakagita, Y. Hayashi, T. Misaka, L-Theanine elicits umami taste via the T1R1 + T1R3 umami taste receptor, Amino Acids 46 [6][2014]1583–1587.
23 D.C. Chu, Green tea – its cultivation, processing of the leaves for drinking materials, and kinds of green tea, in: T. Yamamoto, J.R. Lekh, D.C. Chu, M. Kim [Eds.], Chemistry and Applications of Green Tea, CRC Press, Boca Raton, 1997, pp. 1–11.
24 L.R. Juneja, D.C. Chu, T. Okubo, Y. Nagato, H. Yokogoshi, L-theanine—a unique amino acid of green tea and its relaxation effect in humans, Trend Food Sci. Technol. 10 [12][1999]425.
25 K. Boros, N. Jedlinszki, D. Csupor, Theanine and caffeine content of infusions prepared from commercial tea samples, Pharmacogn. Mag. 12 [45][2016]75–79.
26 J. Williams, J. Kellett, P.D. Roach, A. McKune, D. Mellor, J. Thomas, N. Naumovski, L-theanine as a functional food additive: its role in disease prevention and health promotion, Beverages 2 [13][2016], http://dx.doi.org/10. 3390/beverages2020013.
27 Q.V. Vuong, M.C. Bowyer, P.D. Roach, L-theanine: properties, synthesis and isolation from tea, J. Sci. Food Agric. 91 [11][2011]1931–1939.
28 Lichtenstein N, Preparation of c-alkylamides of glutamic acid. J Am Chem Soc 64:1021–1022 [1942].
29 Kawagishi H and Sugiyama K, Facile and large-scale synthesis of L-theanine. Biosci Biotechnol Biochem 56:689 [1992].
30 Yan SH, Dufour JP and Meurens M, Synthesis and characterization of highly pure theanine. J Tea Sci 23:99–104 [2003].
31. Gu H, Jiang Y and Wang J, A practical synthesis of ethyl L-glutamine [L-theanine]. Org Prep Proced Int 36:182–185 [2004].
32. Zhang F, Zheng QZ, Jiao QC, Liu JZ and Zhao GH, Enzymatic synthesis of theanine from glutamic acid γ -methyl ester and ethylamine by immobilized Escherichia coli cells with γ –glutamyltranspeptidase activity. Amino Acids 39:1177–1182 [2010].
33. Miyake K and Kakita S, A novel catalytic ability of γ –glutamylcysteine synthetase of Escherichia coli and its application in theanine production. Biosci Biotechnol Biochem 73:2677–2683 [2009].
34. Yamamoto S, Wakayama M and Tachiki T, Cloning and expression of Methylovorus mays No. 9 gene encoding γ –glutamylmethylamide synthetase: An enzyme usable in theanine formation by coupling with the alcoholic fermentation system of baker’s yeast. Biosci Biotechnol Biochem 72:101–109 [2008].
35. Zhou X, Zhang Z, Jia X, Wu Y, Luo L and Yin Z, Mn2+ enhances theanine-forming activity of recombinant glutamine synthetase from Bacillus subtilis in Escherichia coli. World J Microb Biotechnol 24:1267–1272 [2008].
36. Suzuki H, Izuka S, Miyakawa N and Kumagai H, Enzymatic production of theanine, an ‘umami’ component of tea, from glutamine and ethylamine with bacterial γ -glutamyltranspeptidase. Enzyme
Microb Technol 31:884–889 [2002].
37. Zhang F, Zheng QZ, Jiao QC, Liu JZ and Zhao GH, Synthesis of theanine from glutamic acid γ -methyl ester and ethylamine catalyzed by Escherichia coli having γ –glutamyltranspeptidase activity. Biotechnol Lett 32:1147–1150 [2010].
38. G.W. Varilek, F. Yang, E.Y. Lee, W.J.S. deVilliers, J. Zhong, H.S. Oz, K.F. Westberry, C.J. McClain, Green tea polyphenol extract attenuates inflammation in interleukin-2-difeicient mice, a model of autoimmunity, J. Nutr. 131 [7][2001]2034–2039.
39. S.M. Chacko, P.T. Thambi, R. Kuttan, I. Nishigaki, Beneficial effects of green tea: a literature review, Chin. Med. 5 [2010]13.
40 Cooper R, Morr´e DJ and Morr´e DM, Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J Altern Complement Med 11:521–528 [2005].
41 Mason R, 200 mg of Zen: L-theanine boosts alpha waves, promotes alert relaxation. Altern Complement Ther 7:91–95 [2001].
42. Nobre AC, Rao A and Owen GN, L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac J Clin Nutr 17:167–168 [2008].
43. Lu K, Gray MA, Oliver C, Liley DT, Harrison BJ, Bartholomeusz CF, et al, The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum Psychopharmacol Clin 19:457–465 [2004].
44. Haskell CF, Kennedy DO, Milne AL, Wesnes KA and Scholey AB, The effects of L-theanine, caffeine and their combination on cognition and mood. Biol Psychol 77:113–122 [2008].
45 Owen GN, Parnell H, Bruin EAD and Rycroft JA, The combined effects of L-theanine and caffeine on cognitive performance and mood. Nutr Neurosci 11:193–198 [2008].
46. Di X, Yan J, Zhao Y, Zhang J, Shi Z, Chang Y, et al, L-Theanine protects the APP [Swedish mutation]transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience 18:778–786 [2010].
47. Liu Q, Duan H, Luan J, Yagasaki K and Zhang G, Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology 59:211–217 [2009].
47. Friedman M, Mackey BE, Kim HJ, Lee IS, Lee KR, Lee SU, et al, Structure–activity relationships of tea compounds against human cancer cells. J Agric Food Chem 55:243–253 [2007].
48. Sugiyama T and Sadzuka Y, Theanine, a specific glutamate derivative in green tea, reduces the adverse reactions of doxorubicin by changing the glutathione level. Cancer Lett 212:177–184 [2004].
49. Sadzuka Y, Sugiyama T, Suzuki T and Sonobe T, Enhancement of the activity of doxorubicin by inhibition of glutamate transporter. Toxicol Lett 123:159–167 [2001].
50. Yokogoshi H and Kobayashi M, Hypotensive effect of γ -glutamylmethylamide in spontaneously hypertensive rats. Life Sci 62:1065–1068 [1998].
51. Rogers PJ, Smith JE, Heatherley SV and Pleydell-Pearce CW, Time for tea: Mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology 195:569–577 [2007].
52. Kurihara S, Shibahara S, Arisaka H and Akiyama Y, Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of L-cystine and L-theanine. J Vet Med Sci 69:1263–1270 [2007].
53. Takagi Y, Kurihara S, Higashi N, Morikawa S, Kase T, Maeda A, et al, Combined administration of L-cystine and L-theanine enhances immune functions and protects against influenza virus infection in aged mice. J VetMed Sci 72:157–165 [2010].
Description
L-Theanine is the major amino acid found in Camellia sinensis, the source of green tea. It is an analog of the excitatory neurotransmitter, glutamate, and thusly, binds to glutamate receptors. L-Theanine can antagonize various glutamate receptor subtypes as well as inhibit glutamine and glutamate transporters, which has been shown to be neuroprotective in animal models of focal cerebral ischemia. Further, L-theanine is reported to increase brain levels of dopamine, serotonin, GABA, nerve growth factor, and brain-derived neurotrophic factor.
Chemical Properties
White Crystalline Solid
Uses
A non-protein amino acid mainly found naturally in the green tea plant. It may have activity in modulating the metabolism of cancer chemotherapeutics agents.
Uses
L-Theanine is a safe and non-toxic photogenic food supplement.
It has been studied as a food additive and functional food in relation to human nutrition.
It has noticeable bioactivities including anti-cerebral ischemia-reperfusion injury, stress-reducing, antitumor, anti-aging, and anti-anxiety activities.
Definition
ChEBI: L-Theanine is a N(5)-alkylglutamine where the alkyl group is ethyl. It has been isolated from green tea. It has a role as a neuroprotective agent, a plant metabolite and a geroprotector. It is a tautomer of a N(5)-ethyl-L-glutamine zwitterion.
benefits
L-Theanine is one of the active ingredients in green tea. It has a variety of physiological functions such as protecting nerves, lowering blood pressure, antioxidant, regulating immune response, improving cognitive ability and calming the mind. Therefore, L-Theanine helps to improve concentration and cognitive ability, improve immune function, reduce stress, relieve anxiety and depression symptoms, and improve sleep. In addition, studies have shown that L-Theanine also helps to alleviate symptoms in patients with high blood pressure and reduces the risk of pancreatic cancer in women.
Biological Activity
Amino acid analog of glutamine and component of green tea. Shown to bind to AMPA, Kainate, NMDA and group I mGlu receptors. Displays neuroprotective effects in vivo . Promotes self-renewal of human embryonic stem cells (hESC).
Biochem/physiol Actions
Theanine is able to bind to AMPA, kainite, and NMDA glycine receptors in rat cortical neurons but with less affinity than glutamic acid. It has been studied as a glutamate transport inhibitor, preventing glutamate uptake by M5076 ovarian sarcoma-bearing mouse cells and increasing the effect of doxorubicin on tumor growth in M5076 mice.
Side effects
Side effects have not been reported. But drinking too much tea may cause: Headaches; Trouble staying asleep; Nausea (feeling like you are going to throw up); Irritability; Stomach pain
Synthesis
Theanine was first chemically synthesised in 1942 by Lichtenstein[28]with a yield of 90 g kg-1 by treating pyrrolidone-5-carboxylic acid with aqueous ethylamine for 20 days at 37 ℃. A number of other synthetic approaches have since been developed including a large-scale production method involving the reaction of γ-benzyl glutamate in the presence of trityl chloride and ethylamine [339 g kg-1][29]and a two-step approach involving initial dehydration of L-glutamic acid to L-pyrrolidone carboxylic acid followed by ring opening in the presence of ethylamine to yield theanine [374 g kg-1][30]. More recently, theanine was produced in four steps starting from commercially available N-phthaloyl-L-glutamic acid, which was dehydrated to the corresponding cyclic anhydride by reaction with acetic anhydride and then the ring was opened by reaction with ethylamine. Subsequent de-protection of the amine unit with hydrazine hydrate gave theanine with a 700 g kg-1 overall yield[31].
In the tea plant, theanine is bio-synthesised from glutamic acid and ethylamine by the enzyme theanine synthetase. However, the enzyme is very labile and cannot be used to produce the amino acid in commercial quantities[32]. Therefore, other methods for the enzymatic synthesis of theanine have been developed using bacterial enzymes such as glutaminase, glutamine synthetase and γ-glutamyl-transpeptidase.
target
NF-kB | TGF-β/Smad | IL Receptor | COX | PGE | CTGF
Drug interactions
There is some evidence L-theanine supplements may lower blood pressure. If you take one or more medications to lower blood pressure, you may want to avoid taking L-theanine. Due to its relaxing and sleep-supporting effects, L-theanine may also have an additive effect with some sedative medications. Avoid taking supplements containing D-theanine as it may prevent the absorption of L-theanine in the body.
Source
The tropical and temperate regions of Asian, African, South American countries are considered the main origin of tea plant [C. sinensis or Thea sinensis]. It is a member of Theaceae family. The majority members of Theaceae family are obtained from India, Sri Lanka, China, and Japan. The physiological properties and colour e.g. black, white, green, yellow or oolong tea strongly depends upon the degree of fermentation and processing conditions[20]. L-theanine is mainly derived from a non-edible mushroom of Xerocomus badius, and C. sinensis. It is an amino acid which accumulates in the leaves of tea like C. sasanqua and C. japonica[21]. In tea, L-theanine is responsible for a strong smell [aroma]in general and in particular it is linked with tea umami taste[22]. From the compositional viewpoint, L-theanine comprises approximately 50% of the tea contents. Whereas, the dry tea contains 1-3% of L-theanine only, this higher or lower ratio of L-theanine can vary depending on several factors i.e. cultivation zone, production season, processing techniques, class of tea, time and type of harvest, etc[27]. Additionally, the harvested tea at the beginning of summer is reported to have more theanine compared with tea harvested in late summer[27]. Moreover, L-theanine concentration also depends on the type/class of tea. According to one study, a specific type of C. sinensis var. Sinensis has more L-theanine contents as compared to the C. sinensis var. Assamica[23].
storage
Room temperature
L-Theanine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| Shaanxi LonierHerb Bio Technology Co Ltd | +86-86-+86-86-029-87551862 +8617702909819 | sales006@ingredients-lonier.com | China | 2605 | 58 |
| Suzhou Yuanrui Bio-Tech Co., Ltd | +86-18106132862 +86-18106132862 | sales02@yuanruibio.com | China | 285 | 58 |
| Chengdu ChenLv Herb Co.,Ltd | +86-13608205856; +undefined13608205856 | maryextract@126.com | China | 127 | 58 |
| Shandong Hanjiang Chemical Co., Ltd | +86-0533-2066820 +8618369939125 | hanson@sdhanjiang.com | China | 1000 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 646 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3957 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5882 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | ada@sh-teruiop.com | China | 882 | 58 | |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
Related articles
- L-Theanine: Food Sources, Effects and Benefits, Side Effects
- L-Theanine is a natural non-protein amino acid extracted from green tea. It is widely used in the food supplement, pharmaceuti....
- Apr 12,2024
- Effectiveness of L-Theaninede
- L-Theanine is the major amino acid found in Camellia sinensis, the source of green tea. L-theanine is reported to increase bra....
- Sep 27,2022
- What is L-theanine good for?Benefits And Use,Dosage
- L-theanine is an amino acid. The human body does not produce this compound, and it is not essential for humans. Green tea, bla....
- Sep 17,2020
View Lastest Price from L-Theanine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | L-Theanine
3081-61-6
|
US $999.00-800.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-20 | L-theanine
3081-61-6
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-09-20 | L-Theanine
3081-61-6
|
US $0.00 / KG | 1KG | ≥98% HPLC | 1000KG | Changsha Staherb Natural Ingredients Co., Ltd. |
-

- L-Theanine
3081-61-6
- US $999.00-800.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- L-theanine
3081-61-6
- US $0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- L-Theanine
3081-61-6
- US $0.00 / KG
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.