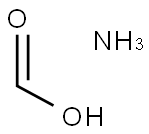
Ammonium formate
- CAS No.
- 540-69-2
- Chemical Name:
- Ammonium formate
- Synonyms
- AMMONIUM FORMAT;AMMONIUM FORMIATE;Ammomium formate;HCOONH4 CH5NO2;mravencanamonny;97.0%, for HPLC;Formiate ammonia;AMMONIUM FORMATE;ammmonium formate;formated’ammonium
- CBNumber:
- CB3140017
- Molecular Formula:
- CH5NO2
- Molecular Weight:
- 63.06
- MDL Number:
- MFCD00013103
- MOL File:
- 540-69-2.mol
- MSDS File:
- SDS
| Melting point | 119-121 °C (lit.) |
|---|---|
| Boiling point | 103.28°C (rough estimate) |
| Density | 1.26 g/mL at 25 °C (lit.) |
| vapor pressure | 0.033Pa at 25℃ |
| refractive index | 1.4164 (estimate) |
| Flash point | 104℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 10 M at 20 °C, clear, colorless |
| form | Solid |
| color | White |
| PH | 6.53(1 mM solution);6.5(10 mM solution);6.49(100 mM solution);6.45(1000 mM solution) |
| Odor | Slight formic acid odour |
| PH Range | 6 - 7 |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.04 |
| Merck | 14,523 |
| BRN | 3625095 |
| Stability | Stable. Hygroscopic. Incompatible with strong acids, strong oxidizing agents. |
| InChIKey | VZTDIZULWFCMLS-UHFFFAOYSA-N |
| LogP | -3.34 at 25℃ |
| FDA 21 CFR | 573.170 |
| CAS DataBase Reference | 540-69-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 3801F9644E |
| EPA Substance Registry System | Ammonium formate (540-69-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | BQ6650000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29151200 | |||||||||
| Toxicity | LD50 orl-mus: 2250 mg/kg ZERNAL 9,332,69 | |||||||||
| NFPA 704 |
|
Ammonium formate price More Price(55)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 78314 | Ammonium formate solution BioUltra, 10?M in H2O | 540-69-2 | 100mL | $107 | 2024-03-01 | Buy |
| Sigma-Aldrich | 78314 | Ammonium formate solution BioUltra, 10?M in H2O | 540-69-2 | 500mL | $373 | 2024-03-01 | Buy |
| Sigma-Aldrich | 70221 | Ammonium formate eluent additive for LC-MS, LiChropur?, ≥99.0% | 540-69-2 | 25g | $170 | 2024-03-01 | Buy |
| Sigma-Aldrich | 70221 | Ammonium formate eluent additive for LC-MS, LiChropur?, ≥99.0% | 540-69-2 | 100g | $617 | 2024-03-01 | Buy |
| Sigma-Aldrich | 156264 | Ammonium formate reagent grade, 97% | 540-69-2 | 1kg | $144 | 2024-03-01 | Buy |
Ammonium formate Chemical Properties,Uses,Production
Description
Ammonium formate is the ammonium salt of formic acid, and is a colorless, hydroscopic, crystalline solid. It can be synthesized through treating ammonium carbonate with 85% formic acid. It is widely used in many organic reactions such as Leuckart reaction which is the reductive amination of aldehydes and ketones. It can also be used as a buffer in HPLC and LC/MS test. Moreover, it is also used in palladium on carbon (Pd/C) reduction of functional group. It can also be used for the preparation of formic acid in situ as well as being used to store formic acid.
References
http://www.orgsyn.org/demo.aspx?prep=CV2P0503
https://www.alfa.com/zh-cn/catalog/014517/
https://en.wikipedia.org/wiki/Ammonium_formate
Description
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. It is a colorless, hygroscopic, crystalline solid.
Chemical Properties
colourless crystals
Physical properties
White monoclinic deliquescent crystals or granules; density 1.280 g/cm3; melts at 116°C; highly soluble in water (102 g/100 g at 0°C), solubility rapidly increasing with temperature (i.e., 531 g/100 g at 80°C); soluble in liquid ammonia, alcohol and ether.
Uses
Pure ammonium formate decomposes into formamide and water when heated, and this is its primary use in industry. Formic acid can also be obtained by reacting ammonium formate with a dilute acid, and since ammonium formate is also produced from formic acid, it can serve as a way of storing formic acid.
Ammonium formate can also be used in palladium on carbon (Pd / C) reduction of functional groups. In the presence of Pd / C, ammonium formate decomposes to hydrogen, carbon dioxide, and ammonia.
Ammonium formate can be used for reductive amination of aldehydes and ketones (Leuckart reaction)
Ammonium formate can be used as a buffer in high performance liquid chromatography (HPLC), and is suitable for use with liquid chromatography/mass spectrometry (LC/MS). .
Uses
Ammonium formate is widely used in various organic reactions like Leuckart reaction which involves the reductive amination of aldehydes and ketones. It serves as a buffer in high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC/MS). It finds application in palladium on carbon (Pd/C) reduction of functional groups. For example, reduction of alkenes to alkanes and formaldehyde to methanol. It is also used to prepare formic acid insitu as well as used to store formic acid by making it as an ammonium salt.
Uses
In chemical analysis, especially to ppt base metals from salts of the "noble" metals.
Definition
ChEBI: The ammonium salt of formic acid.
Reactions
When heated, ammonium formate eliminates water, forming formamide. Upon further heating it forms to HCN and H2O. A side reaction of this is the decomposition of formamide to CO and NH3.
General Description
White solid with a weak odor of ammonia. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as Ammonium formate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion irritates mouth and stomach. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating ammonia and formic acid gases may form in fire.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by ingestion andintravenous routes. When heated to decomposition itemits toxic fumes of NOx and NH3.
Purification Methods
Heat the solid in NH3 vapour and dry it in a vacuum till the NH3 odour is faint (note that it can evaporate completely in a vacuum). Recrystallise it from absolute EtOH and then keep it in a desiccator over 99% H2SO4 in vacuo. It is very hygroscopic. It exists in two forms, stable needles and less stable plates. It also forms acid salts, i.e. HCO2NH4.3HCO2H and HCO2NH4.HCO2H. [Kensall & Adler J Am Chem Soc 43 1473 1921, Beilstein 2 IV 18.]
Ammonium formate Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34571 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 | sales3257@jskolod.com | China | 132 | 60 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3878 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 | Ethan@dornechem.com | China | 3138 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5882 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 | admin@juchuangchem.com | China | 387 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1143 | 58 |
Related articles
- Ammonium formate: Applications as Electrochemical Fuel Ionic Liquid and Safety
- Ammonium formate shows promise as an electrochemical fuel, but safety concerns arise in animal feed due to formamide presence,....
- May 27,2024
- The toxicity of Ammonium formate
- Ammonium formate (E 295) is presently listed in the EU Register of Feed Additives as a technological additive (functional grou....
- May 13,2024
- Ammonium formate: Applications and reactions
- Ammonium formate is obtained by neutralizing formic acid and ammonia. It is soluble in water and ethanol, and its aqueous solu....
- May 23,2023
View Lastest Price from Ammonium formate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | Ammonium formate
540-69-2
|
US $1.00 / g | 1g | 99% | 1000kg | Dorne Chemical Technology co. LTD | |
 |
2024-09-19 | Ammonium Formate
540-69-2
|
US $11.00 / kg | 1kg | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-18 | Ammonium formate
540-69-2
|
US $0.00 / Kg/Bag | 25KG | 98%min | 20 TONS | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Ammonium formate
540-69-2
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Ammonium Formate
540-69-2
- US $11.00 / kg
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Ammonium formate
540-69-2
- US $0.00 / Kg/Bag
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD