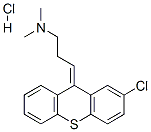
Chlorprothixene hydrochloride
- CAS No.
- 6469-93-8
- Chemical Name:
- Chlorprothixene hydrochloride
- Synonyms
- NSC 78193;NSC 56379;NSC 169899;Minithixen;CHLORPROTHIXENE HCL;Truxal Hydrochloride;Chlorprothixene HCI BP;Minithixen hydrochloride;CHLORPROTHIXENE HYDROCHLORIDE;cis-chlorprothixenehydrochloride
- CBNumber:
- CB3325953
- Molecular Formula:
- C18H19Cl2NS
- Molecular Weight:
- 352.32
- MDL Number:
- MFCD01941605
- MOL File:
- 6469-93-8.mol
- MSDS File:
- SDS
| Melting point | 224-226?C |
|---|---|
| storage temp. | 2-8°C |
| solubility | Soluble in water and in alcohol, slightly soluble in methylene chloride. |
| CAS DataBase Reference | 6469-93-8 |
| FDA UNII | 268KCR965N |
Chlorprothixene hydrochloride price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C1915000 | Chlorprothixene hydrochloride European Pharmacopoeia (EP) Reference Standard | 6469-93-8 | c1915000 | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | C1671 | Chlorprothixene hydrochloride | 6469-93-8 | 1g | $38.9 | 2024-03-01 | Buy |
| Cayman Chemical | 20772 | Chlorprothixene (hydrochloride) ≥98% | 6469-93-8 | 1g | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 20772 | Chlorprothixene (hydrochloride) ≥98% | 6469-93-8 | 5g | $139 | 2024-03-01 | Buy |
| Cayman Chemical | 20772 | Chlorprothixene (hydrochloride) ≥98% | 6469-93-8 | 10g | $243 | 2024-03-01 | Buy |
Chlorprothixene hydrochloride Chemical Properties,Uses,Production
Chemical Properties
Pale Yellow Solid
Originator
aractan, Roche, France ,1960
Uses
Neuroleptic;D2 dopamine receptor antagonist
Uses
Antipsychotic.
Uses
Chlorprothixene hydrochloride has been used to study its exposure effects on learning and memory of rats.
Manufacturing Process
Chlorprothixene may be prepared as described in US Patent 2,951,082.
Magnesium turnings, 4.86 g (0.2 g-atom) was placed in a 500 ml reaction
flask fitted with a mercury sealed stirrer, reflux condenser and a dropping
funnel. Tetrahydrofuran, 50 ml and calcium hydride, 500 mg, were added.
Ethyl bromide, 2.18 g and a crystal of iodine then were added. A vigorous
reaction set in that evolved sufficient heat to induce refluxing. After 5
minutes, a solution of 3-dimethylaminopropyl chloride (dried over calcium
hydride) in 50 ml of tetrahydrofuran was added to the refluxing solution at
such a rate that gentle refluxing was maintained. The addition required 25
minutes.
The reaction mixture was stirred at reflux for an additional 30 minutes when
nearly all of the magnesium had dissolved and determination of magnesium in
an aliquot of the solution showed that an 82% yield of Grignard reagent had
been obtained. The reaction mixture was cooled in an ice bath and stirred
while 24.67 g (0.1 mol) of 2-chlorothiaxanthone was added over a period of
10 minutes. The reaction was stirred at room temperature for 30 minutes
then allowed to stand overnight in the refrigerator. The tetrahydrofuran was
evaporated at 50°C under reduced pressure. Benzene, 150 ml, was added to
the residue.
The mixture was hydrolyzed in the cold by the dropwise addition of 50 ml of
water. The benzene layer was separated by decantation and the gelatinous
precipitate washed with two 100 ml portions of benzene.
The precipitate was then mixed with diatomaceous earth, collected on a filter,
and washed with water and extracted with two 100 ml portions of boiling
benzene. The aqueous filtrate was extracted with 50 ml of benzene, the
combined benzene extracts washed with water and evaporated to dryness
under reduced pressure. The crystalline residue, MP 140° to 147°C, weighed
30.8 g. Recrystallization from a mixture of benzene and hexane gave 27.6 g
(83%) of 2-chloro-10-(3-dimethylaminopropyl)-10-hydroxythiaxanthene, MP
152° to 154°C. Analytically pure material from another experiment melted at
153° to 154°C.
2-Chloro-10-(3-dimethylaminopropyl)-10-hydroxythiaxanthene, 3.34 g (0.01
mol) obtained as described was dissolved in 15 ml of dry, alcohol-free
chloroform. Acetyl chloride, 2.36 g (0.03 mol) was added and the clear yellow
solution was refluxed for one hour in a system protected by a drying tube.
The solvent then was evaporated on the steam bath under reduced pressure
and the residue dissolved in absolute alcohol. The hydrochloride of 2-chloro-
10-(3-dimethylaminopropylidene)-thiaxanthene was precipitated by the
cautious addition of absolute ether. After drying at 70°C the yield of white
crystalline 2-chloro10-(3-dimethylaminopropylidene)-thiaxanthene
hydrochloride, MP 189 to 190°C (to a cloudy melt), was 3.20 g (90%). This
material is a mixture of geometric isomers.
Trans-2-chloro-9-(ω-dimethylamino-propylidene)-thioxanthene [MP 98°C, MP
of the hydrochloride 225°C (corr.)], is a valuable medicinal agent, being used
as a tranquilizer and antiemetic agent, whereas the corresponding cis isomer
(MP 44°C, MP of the hydrochloride 209°C) is not useful for these indications,
as described in US Patent 3,115,502, which describes procedures for
conversion of the cis to the trans form.
Therapeutic Function
Tranquilizer
General Description
Chlorprothixene is a neuroleptic drug, which belongs to thioxanthene group. It has anticholinergic effects. Chlorprothixene might be associated with obstructive jaundice and parkinsonism. It is used to treat psychosis.
Biological Activity
chlorprothixene (hydrochloride) is an antagonist of dopamine receptor and histamine receptors [1]. chlorprothixene is also an inhibitor of gabaa receptor [2]. all of these three receptors are implicated in many neurological processes, including motivation, pleasure, cognition, memory and learning.
in vitro
chlorprothixene exihibited strong binding affinities to dopamine and histamine receptors, the ki values of d1, d2, d3, d5 and h1 were 18nm, 2.96 nm, 4.56 nm, 9 nm and 3.75 nm, respectively. chlorprothixene showed little affinity to h3 with the ki value of >1000 nm[1]. in cos-7 cells transiently expressed rat 5-ht7 receptors and hek-293 cells stably transfected with rat 5-ht6, the ki values of chlorprothixene were 5.6 nm and 3 nm, respectively [3]. in vero 76 cells, chlorprothixene treatment inhibited sars-cov replication, with ic50 of 16.7 μm for urbani strain, 13.0 μm for frankfurt-1, 18.5 μm for chuk-w1 and 15.8 μm for toronto-2 [4].
in vivo
in rat brain depressing the release of hypothalamic and hypophyseal hormones, chlorprothixene blocked postsynaptic mesolimbic dopaminergic d1 and d2 receptors [5]. chlorprothixene treatment restored normal ceramide concentrations in murine bronchial epithelial cells, reduced inflammation in the lungs of mice with cystic fibrosis (cf) and prevented infection with pseudomonas aeruginosa [6].
References
[1] von coburg y, kottke t, weizel l, et al. potential utility of histamine h 3 receptor antagonist pharmacophore in antipsychotics[j]. bioorganic & medicinal chemistry letters, 2009, 19(2): 538-542.
[2] squires r f, saederup e. clozapine and several other antipsychotic/antidepressant drugs preferentially block the same ‘core’fraction of gaba a receptors[j]. neurochemical research, 1998, 23(10): 1283-1290.
[3] roth b l, craigo s c, choudhary m s, et al. binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors[j]. journal of pharmacology and experimental therapeutics, 1994, 268(3): 1403-1410.
[4] barnard d l, day c w, bailey k, et al. is the anti-psychotic, 10-(3-(dimethylamino) propyl) phenothiazine (promazine), a potential drug with which to treat sars infections: lack of efficacy of promazine on sars-cov replication in a mouse model[j]. antiviral research, 2008, 79(2): 105-113.
[5] gey k f, pletscher a. influence of chlorpromazine and chlorprothixene on the cerebral metabolism of 5-hydroxytryptamine, norepinephrine and dopamine[j]. journal of pharmacology and experimental therapeutics, 1961, 133(1): 18-24.
[6] becker k a, riethmuller j, luth a, et al. acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis[j]. american journal of respiratory cell and molecular biology, 2010, 42(6): 716-724.]
Chlorprothixene hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2255 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 28353 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| Hefei Hirisun Pharmatech Co., Ltd | +8615056975894 | shawn@hirisunpharm.com | CHINA | 9923 | 58 |
View Lastest Price from Chlorprothixene hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-24 | Chlorprothixene hydrochloride USP/EP/BP
6469-93-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2020-02-19 | 2-Chloro-9-(3-dimethylaminopropylidene)thioxanthene hydrochloride
6469-93-8
|
US $1.00 / KG | 1KG | 98% HPLC | 10 tons | Career Henan Chemical Co |
-

- Chlorprothixene hydrochloride USP/EP/BP
6469-93-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- 2-Chloro-9-(3-dimethylaminopropylidene)thioxanthene hydrochloride
6469-93-8
- US $1.00 / KG
- 98% HPLC
- Career Henan Chemical Co