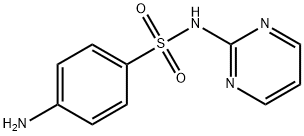
Sulfadiazine
- CAS No.
- 68-35-9
- Chemical Name:
- Sulfadiazine
- Synonyms
- SULPHADIAZINE;Sulfadiazine,(S);SULFADIAZIN;Sulfose;Terfonyl;Diazolone;Neotrizine;Sulfadiazene;SULPHADIAZINE BP;-AMino-N-2-pyriMidinylbenzenesulfonaMide
- CBNumber:
- CB4166214
- Molecular Formula:
- C10H10N4O2S
- Molecular Weight:
- 250.28
- MDL Number:
- MFCD00006065
- MOL File:
- 68-35-9.mol
- MSDS File:
- SDS
| Melting point | 253 °C (dec.) (lit.) |
|---|---|
| Boiling point | 512.6±52.0 °C(Predicted) |
| Density | 1.3780 (rough estimate) |
| refractive index | 1.6440 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in dimethyl sulfoxide. |
| pka | pKa 2.21(H2O t = 25 I = 0.5 (NaCl)) (Uncertain) |
| form | powder |
| color | white |
| Water Solubility | 67.13mg/L(25 ºC) |
| Merck | 14,8903 |
| BRN | 6733588 |
| BCS Class | 4/3 |
| CAS DataBase Reference | 68-35-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 0N7609K889 |
| ATC code | J01EC02 |
| NIST Chemistry Reference | Sulfadiazine(68-35-9) |
| EPA Substance Registry System | Benzenesulfonamide, 4-amino-N-2-pyrimidinyl- (68-35-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H361d-H362-H411 | |||||||||
| Precautionary statements | P202-P260-P263-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-42/43-43-42 | |||||||||
| Safety Statements | 26-36 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WP1925000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29335990 | |||||||||
| Toxicity | LD50 oral in mouse: 1500mg/kg | |||||||||
| NFPA 704 |
|
Sulfadiazine price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | BP312 | Sulfadiazine British Pharmacopoeia (BP) Reference Standard | 68-35-9 | 100MG | $266 | 2024-03-01 | Buy |
| Sigma-Aldrich | 35033 | Sulfadiazine VETRANAL , analytical standard | 68-35-9 | 100mg | $60.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1625009 | Sulfadiazine United States Pharmacopeia (USP) Reference Standard | 68-35-9 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | S0579 | Sulfadiazine >99.0%(HPLC)(T) | 68-35-9 | 25g | $36 | 2024-03-01 | Buy |
| Alfa Aesar | A12370 | Sulfadiazine, 99% | 68-35-9 | 50g | $28.4 | 2024-03-01 | Buy |
Sulfadiazine Chemical Properties,Uses,Production
description
Sulfadiazine is an oral sulfonamide anti-bacterial agent. it is a synthetic pyrimidinyl sulfonamide derivative, The chemical classification of sulfadiazine is Sulfonamides.The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus.
Sulfadiazine is used in combination with pyrimethamine to treat toxoplasmosis in patients with acquired immunodeficiency syndrome and in newborns with congenital infections.
Chemical Properties
It is White or white-like crystal or powder and is odorless and tasteless. It gradually becomes dark upon exposure to light. It is insoluble in water, soluble in boiling water (1:60), slightly soluble in ethanol and acetone and insoluble in chloroform and ether. It is easily soluble in dilute hydrochloric acid, sodium hydroxide solution or ammonia solution. Its melting point is 252~256 ℃ (decomposition occurs at the same time). Its sodium salt is a white crystalline powder and is odorless with slightly bitter taste. Its sodium salt gradually turns to brown color up exposure to light. When being subject to long-term storage in the moist air, it can slowly absorb carbon dioxide and precipitate out sulfadiazine. It is soluble in water, slightly soluble in ethanol, but insoluble in chloroform and ether. Its 10% aqueous solution has a pH of 9.5 to 10.5.
Pharmacological effects
Sulfadiazine hemolytic has inhibitory effect on various kinds of microorganisms including streptococcus, staphylococcus, meningococcus, pneumococcus, Neisseria gonorrhoeae, Escherichia coli, Shigella and other sensitive bacteria as well as Chlamydia trachomatis, actinomycetes, Plasmodium, Toxoplasma gondii and Star Nocardia. The antibacterial activity of this product is similar as that of sulfasuccinamide. But in recent years, there are increased reports regarding the bacterial resistance to this product, especially in Streptococcus, Neisseria and Enterobacteriaceae. Sulfa-class belongs to broad-spectrum antibacterial agents. The molecular structure of sulfadiazine is similar as that of the amino benzoic acid (PABA), and can compete with PABA for acting on the dihydrofolate synthetase inside bacterial cells, thereby preventing the biosynthesis of folic acid (essential for bacteria) using PABA as the raw material and further reducing the amount of metabolically active folate, which is a indispensible material for bacterial synthesis of purines, thymidine and deoxyribonucleic acid (DNA) and thereby inhibiting the growth and reproduction of bacteria.
Pharmacokinetics
This product can be easily absorbed after oral administration (more than 70% of the administrated drug can be absorbed), but with the absorption rate being relative slow. After a single oral dose of 2g, the plasma concentration reaches peak after 3~6 hours with the peak of free plasma concentration being about 30~60μg/ml. After drug absorption, it widely distributed in body tissue and pleural fluid, peritoneal fluid, synovial fluid, aqueous humor, saliva, sweat, urine and bile. The drug is easy to penetrate through the blood-brain barrier as well as being able to enter into the breast milk and penetrate through the placental barrier. When there is no meningeal inflammation, cerebrospinal fluid drug concentration is about 50% of the plasma concentration. While the value can be 50% to 80% when there is meningeal inflammation.
The drug has a low plasma protein binding rate which is around 38% to 48%. The elimination life for patients of normal renal function is about 10 hours while it can be as long as 34 hours for patients with kidney failure. Sulfadiazine drug is mainly deactivated in the liver through acetylation metabolism, followed by deactivation upon binding to the glucuronide in the liver. The drug is primarily excreted through glomerular filtration. Within 48 to 72 hours after administration of the drug, around 60% to 85% of administrated drug is excreted form urine. In addition, there is still a small amount of drugs being able to be discharged through feces, milk, and bile. Hemodialysis can partially clear the drug. However, peritoneal dialysis can’t effectively remove the drugs.
Indications
Sulfa drugs belong to broad-spectrum antibiotic. However, because of the resistance of many current clinical common pathogens to this class of drugs, it is only used for treating the infection caused by sensitive bacteria and other kinds of susceptible pathogens.
Sulfadiazine (not including the FDC of this drug together with trimethoprim) has its main indications as follows:
1. Used for prevention and treating the meningococcal meningitis caused by sensitive Meningococcal.
2. When being used in combination with trimethoprim, it can be used for treating the otitis media and other kinds of soft tissue infection caused sensitive Haemophilus influenzae, Streptococcus pneumoniae and other kinds of Streptococcus.
3. Used for treating disease related to star nocardia.
4. Used as the adjuvant drug assisting in the treatment of chloroquine-resistant falciparum malaria.
5. Used as the secondary-choice drug for treatment of Chlamydia trachomatis-induced urethritis and cervicitis.
6. Used as the secondary-choice drug for treatment of neonatal inclusion conjunctivitis caused by Chlamydia trachomatis.
Side effects
1. it can cause kidney damage. This product has a low acetylation ratio. This product and its acetylated compound has a low solubility in the urine and is easily subject to crystal precipitation upon a acidic urine, doing harm to the renal tubular as well as the epithelial cells of other urinary tract and causing crystallization of urine, hematuria, proteinuria, and even urine retention or uremia in severe cases.
2. hematopoietic system reactions include neutropenia, acute hemolytic anemia, aplastic anemia, and thrombocytopenia purpura.
3. gastrointestinal reactions include nausea and vomiting. Occasionally: jaundice, liver and spleen. For newborns, premature children, it can cause jaundice, and even kernicterus.
4. urinary system damage: As the prototype of sulfonamides and acesulfame are primarily subject to renal excretion and thus have higher concentrations in the urine. Upon acidic urine, its solubility decreases and can be crystallized and precipitated in renal tubules, renal pelvis, ureter or bladder, causing crystallization of urine, hematuria, proteinuria, dysuria, oliguria and even urine retention.
5. allergic reactions: commonly include rash, drug fever and even exfoliative dermatitis, erythema multiforme exudativum in severe cases. This often occurs during the 7 to 10 days after the medication. Photosensitive dermatitis has also been reported.
Uses
It is excellent kinds of sulfa drugs with strong antibacterial activity, good efficacy, rapid absorption, slow excretion rate and high plasma concentration. It is clinically used for treating upper respiratory tract infection, Meningococcal meningitis, otitis media, boils carbuncle, puerperal fever, urinary tract infections and acute dysentery and so on.
It is a sulfa-type drug which is used for the treatment of infection caused by hemolytic streptococcus, pneumococcus, meningococcis, Neisseria gonorrhea, and E. coli.
It is a sulfa drug with antibacterial effect and convergence effect.
Chemical Properties
White to slightly yellow crystalline pow
Originator
Sulfadiazine,Lederle,US ,1941
Uses
DMSO soluble potent immunosuppressant, neuroprotective neuroregenerative, in vitro T cell proliferation blocker. disrupts calcineurin-mediated signal transduction in T lymphocytes
Uses
Sulfonamide antibacterial.
Uses
It is used in the form of silver salts (sulfadiazine silver) as an external antibacterial agent, primarily for treating burns. It is believed that the presence of the silver ion in the molecule facilitates increased antimicrobial and wound-healing action.
Definition
ChEBI: A sulfonamide consisting of pyrimidine with a 4-aminobenzenesulfonamido group at the 2-position.
Manufacturing Process
5.4 parts of 2-amino-pyrimidine were covered with 15 parts of anhydrous
pyridine. The reaction mixture was treated with 14 parts of pnitrobenzenesulfonyl
chloride and the whole heated briefly on the steam bath
and let stand 45 minutes at room temperature. To the reaction mixture were
added 80 parts of hot alcohol and the precipitate was filtered off and washed
with water. The solid was dissolved in dilute caustic solution and the solution
was filtered, cooled and acidified. The 2-(p-nitrobenzenesulfonamido)-
pyrimidine precipitated and was collected.
The crude 2-(p-nitrobenzenesulfonamido)-pyrimidine from the preceding step
was suspended in 130 parts alcohol and 1.5 parts of concentrated hydrochloric
acid were added. The suspension was then heated to reflux and 30 parts of
iron powder were added with mechanical stirring. The mixture was refluxed
and stirred for 24 hours with occasional addition of concentrated hydrochloric
acid. The reaction mixture was then made slightly basic and filtered hot and
the residues were extracted with several portions of boiling alcohol. The filtrate and wash solutions were combined and evaporated. The 2-
(sulfanilamido)-pyrimidine was recrystallized from boiling water with
decolorizing charcoal added, according to US Patent 2,410,793.
brand name
Coco-Diazine (Lilly); Eskadiazine (SmithKline Beecham).
Therapeutic Function
Antibacterial
Antimicrobial activity
Sulfadiazine is somewhat more active than other sulphonamides.
General Description
Sulfadiazine’s plasma half-life is 17 hours. It is a white,odorless crystalline powder soluble in water to the extentof 1:8,100 at 37°C and 1:13,000 at 25°C, in human serumto the extent of 1:620 at 37°C, and sparingly soluble in alcoholand acetone. It is readily soluble in dilute mineralacids and bases. Its pKa is 6.3.
Pharmaceutical Applications
Sulfadiazine is almost insoluble in water and unstable on exposure to light. It is administered orally or, as the sodium salt, by intravenous injection. It is a component of several multi-ingredient preparations. Its low solubility in urine led to its general replacement by other compounds. The intravenous solution is highly alkaline and should not be given by any other route.
Biochem/physiol Actions
Sulfadiazine is a sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase. Sulfadiazine is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), which is required for bacterial synthesis of folic acid. It is active against Gram positive bacteria, Gram negative bacteria and Chlamydia. Mode of resistance is via the alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis.
Pharmacokinetics
Oral absorption: Very good
Cmax 3 g oral: c. 50 mg/L after 3–4 h
Plasma half-life :7–12 h
Volume of distribution: 0.36 L/kg
Plasma protein binding: c. 40%
Absorption and distribution
Adequate blood concentrations are easily achieved and
maintained after oral administration. It is well distributed
and penetrates in therapeutic concentrations into
the CSF, but because of resistance it is no longer the
drug of choice in meningitis. It crosses the placenta and
enters breast milk to achieve concentrations around 20%
of plasma levels.
Metabolism and excretion
Sulfadiazine is subject to acetylation in the liver. The acetyl
derivative lacks antibacterial activity and is excreted more
slowly (half-life 8–18 h). Parent compound and metabolite
are both excreted mainly by glomerular filtration.
Clinical Use
Urinary tract infection
Nocardiasis
Chancroid
Toxoplasmosis (in combination with pyrimethamine)
Meningococcal infections
Prophylaxis of rheumatic fever
Side effects
In addition to side effects common to the group, sulfadiazine inhibits the metabolism of phenytoin. The risk of crystalluria can be reduced by high fluid intake and alkalization of the urine.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by ingestion: hematuria, anuria, general anesthesia, gastrointestinal effects. Experimental teratogenic and reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and SOx.
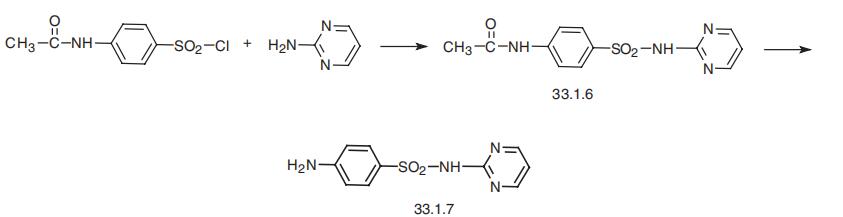
Synthesis
Sulfadiazine, N1 -2-pyrimidinylsulfanilamide (33.1.7), is synthesized by reacting 4-acetylaminobenzenesulfonyl chloride with 2-aminopyrimidine, which gives an acetanilide derivative (33.1.6). The subsequent hydrolysis of this product with a base leads to the formation of the desired sulfadiazine.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: increased risk of crystalluria with
methenamine.
Anticoagulants: effect of coumarins enhanced;
metabolism of phenindione possibly inhibited.
Antiepileptics: antifolate effect and concentration of
phenytoin increased.
Antimalarials: increased risk of antifolate effect with
pyrimethamine.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis).
Ciclosporin: reduced levels of ciclosporin; increased
risk of nephrotoxicity.
Cytotoxics: increase risk of methotrexate toxicity
Metabolism
Sulfadiazine is metabolised in the liver to the acetylated form, with elimination predominantly via the kidneys. Urinary excretion of sulfadiazine and its acetyl derivative is dependent on pH; when the urine is acidic about 30% is excreted unchanged in both fast and slow acetylators, whereas when the urine is alkaline about 75% is excreted unchanged by slow acetylators.
Sulfadiazine Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5866 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1534 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1143 | 58 |
| Apeloa production Co.,Limited | +8619933239880 | admin@apcl.com.cn | China | 853 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +86-18239973690 +86-18239973690 | sales@suikangpharm.com | China | 311 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Shengyang Water Conservancy Engineering Co., Ltd. | +8615373025980 | clara@hbshengyang.com | China | 874 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
View Lastest Price from Sulfadiazine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-19 | Sulfadiazine
68-35-9
|
US $20.00 / kg | 10kg | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-18 | Sulfadiazine
68-35-9
|
US $0.00-0.00 / Kg/Drum | 1KG | 99%-101%; BP | 1000 KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-09-06 | Sulfadiazine
68-35-9
|
US $0.00 / kg | 25kg | 99% | 10tons | Henan Suikang Pharmaceutical Co.,Ltd. |
-

- Sulfadiazine
68-35-9
- US $20.00 / kg
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Sulfadiazine
68-35-9
- US $0.00-0.00 / Kg/Drum
- 99%-101%; BP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Sulfadiazine
68-35-9
- US $0.00 / kg
- 99%
- Henan Suikang Pharmaceutical Co.,Ltd.