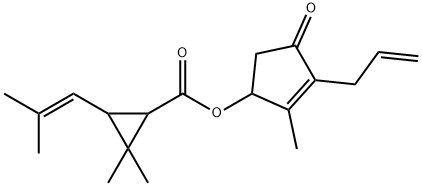
Allethrin
- CAS No.
- 584-79-2
- Chemical Name:
- Allethrin
- Synonyms
- Esbioi;fmc249;nia249;oms468;D-TRANS;exthrin;fda1446;FMC 249;NIA 249;OMS 468
- CBNumber:
- CB6681269
- Molecular Formula:
- C19H26O3
- Molecular Weight:
- 302.41
- MDL Number:
- MFCD00045443
- MOL File:
- 584-79-2.mol
- MSDS File:
- SDS
| Melting point | approximate 4℃ |
|---|---|
| Boiling point | 160°C |
| Density | 1.01 |
| vapor pressure | 1.6×10-4 Pa (21 °C) |
| refractive index | nD20 1.5040; nD30 1.5023 |
| Flash point | 66 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform: Slightly Soluble |
| Water Solubility | 2 mg l-1 |
| form | Viscous Liquid |
| color | Clear amber |
| BRN | 2294836 |
| LogP | 4.780 |
| CAS DataBase Reference | 584-79-2(CAS DataBase Reference) |
| EWG's Food Scores | 3 |
| FDA UNII | 0X03II877M |
| NIST Chemistry Reference | Bioallethrin(584-79-2) |
| EPA Substance Registry System | Allethrin (584-79-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H332-H410 | |||||||||
| Precautionary statements | P273-P301+P312+P330-P304+P340+P312 | |||||||||
| Hazard Codes | Xn;N,N,Xn | |||||||||
| Risk Statements | 20/22-50/53-40 | |||||||||
| Safety Statements | 36-60-61-36/37-24/25-23 | |||||||||
| RIDADR | UN 3082 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GZ1925000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29183000 | |||||||||
| Toxicity | LD50 oral in rabbit: 4290mg/kg | |||||||||
| NFPA 704 |
|
Allethrin price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 33309 | Esbiothrin PESTANAL?,analyticalstandard | 584-79-2 | 100mg | $64.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 31489 | Bioallethrin PESTANAL | 584-79-2 | 250mg | $40.6 | 2022-05-15 | Buy |
| Cayman Chemical | 24132 | Allethrin ≥95% | 584-79-2 | 50mg | $36 | 2024-03-01 | Buy |
| Cayman Chemical | 24132 | Allethrin ≥95% | 584-79-2 | 100mg | $66 | 2024-03-01 | Buy |
| Sigma-Aldrich | 33396 | Allethrin mixture of stereo isomers | 584-79-2 | 100mg | $70.6 | 2024-03-01 | Buy |
Allethrin Chemical Properties,Uses,Production
Chemical Properties
Yellow Oil
Uses
Allethrin is a synthetic pyrethroid derivative used as an insecticide. Allethrin is commonly used in many household insecticide products due to its low toxicity towards humans.
Uses
Esbiothrin may be used as a reference standard for the determination of the analyte in electric-mosquito coils using gas chromatography (GC) technique.
Uses
The allethrins are used to control a wide range of insects in horticultural, household, public health and animal health situations. They have some limited use on ornamentals and vegetables (in combination with synergists).
Definition
Generic name for 2-allyl4-hydroxtcyclopenten-1-one ester of chrysanthemummonocarboxylic acid. A synthetic insecticide structurally similar to pyrethrin and used in the same manner. For other synthetic analogs, see barthrin, cyclethrin, ethythrin, furethrin. Pyrethrin I differs in having a 2,4-pentadienyl group in place of the allyl of allethrin.
General Description
A clear amber-colored viscous liquid. Insoluble and denser than water. Toxic by ingestion, inhalation, and skin absorption. A synthetic household insecticide that kills flies, mosquitoes, garden insects, etc.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Allethrin is an ester and ketone. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Agricultural Uses
Insecticide: Allethrin is used almost exclusively to control flying and crawling insects in homes and industrial locations. Used extensively in pet animal shampoos, to treat lice in humans and in home and industrial sprays for flying insects, mosquitos, etc. It is available as mosquito coils, mats, oil formulations and as an aerosol spray. It may be hazardous to the environment; special attention should be given to fish and honey bees. Not currently registered in the U.S. Not approved for use in EU countries. Depending on CAS registry number there are probably >100 global suppliers.
Trade name
BIOALLETHRIN®; BIOALLETHRIN TECHNICAL®; d-CISALLETHRIN®; ESBIOTHRIN®; EXTHRIN® FMC 249®; NIA 249®; OMS 468®; PYNAMIN®; PYNAMIN-FORTE®; PYRESIN®; PYRESYN®; PYREXCEL®; PYROCIDE®; SBP 1382/ BIOALLETHRIN CONCENTRATE®
Contact allergens
Pyrethroids, also called pyrethrinoids, are neurotoxic synthetic compounds used as insecticides, with irritant properties. Cypermethrin and fenvalerate have been reported as causing positive allergic patch tests, but only fenvalerate was relevant in an agricultural worker.
Safety Profile
Poison by intravenous, intracerebral, and intraperitoneal routes. Moderately toxic by ingestion and skin contact. An allergen. An insecticide. It can cause liver and kidney damage by all routes of entry into the body. Lung congestion may occur due to exposure. Local contact may cause contact dermatitis. Inhalation may cause asthma, coughing, wheezing, running nose and eyes. Mutation data reported. See also ALLYL COMPOUNDS and ESTERS. Slight fire hazard. When heated to decomposition it emits acrid fumes.
Metabolic pathway
Allethrin was the first synthetic pyrethroid. Its stereochemistry is RS(cyclopentenyl)lRcis-trans. It was further developed as bioallethrin (RSlRtvuns) and then as S-bioallethrin (SlRtvuns), the most potent of the three. All are very sensitive to light and are used almost entirely indoors. Thus, there is only a limited amount of information on their environmental fate published in the literature. Information on photodegradation and on metabolism in insects and rodents has been reported.
Degradation
The allethrins are reasonably stable but they are sensitive to base
hydrolysis forming chrysanthemic acid (2) and 3-allyl-2-methyl-4-
oxocyclopent-2-enol(3, allethrolone). The DT50 of bioallethrin in aqueous
solution is 547 days at pH 7 and 4.3 days at pH 9.
The allethrins are also sensitive to oxidation and to photodecomposition.
Allethrin was converted almost quantitatively into the
cycloproprethronyl derivative (4) by a di-π-methane rearrangement on
irradiation in hexane solution (Bullivant and Pattenden, 1976). In
addition, isomerisation, oxidation and epoxidation of the isobutenyl
group of the chrysanthemic acid moiety by reactions analogous to those
described under phenothrin have been described (Ruzo et al., 1980).
Most products retained the ester linkage but the acid 2 was identified.
A novel product, 1-cyclopropyl-5-methyl-6-oxabicyclo[3.0.1]hexan-2-on-
4-yl chrysanthemate (5), was then reported (Kimmel et al., 1982). This
product proved to be a potent bacterial mutagen (Ames assay). Later
Isobe et al. (1984) reported photo-oxidation products derived from the
alcohol moiety: the alcohol (3) and allethronyl glyoxalate (6). The
latter was mostly present as its hydrate (7).T he ester (8) was also detected
but this was shown not to be a precursor of 6 or 7. These products
are illustrated in Scheme 1. Products formed by oxidation of the acid
moiety and retaining the ester link, such as 9, 10 and 11, are shown in
Scheme 2.
These two sites of weakness in allethrin render it one of the most photolabile
of the synthetic pyrethroids (Ruzo, 1982) and result in a complex
mixture of degradation products.
Allethrin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 | admin@86-ss.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29822 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
View Lastest Price from Allethrin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-25 | Allethrin
584-79-2
|
US $999.00-666.00 / ton | 0.001ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-08-15 | allethrin
584-79-2
|
US $0.00-0.00 / kg | 1kg | 0.99 | 100tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-01-31 | Allethrin
584-79-2
|
US $50.00 / KG | 1KG | 99% | 5000KG | Hebei Mojin Biotechnology Co., Ltd |