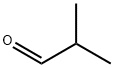
Isobutyraldehyde
- CAS No.
- 78-84-2
- Chemical Name:
- Isobutyraldehyde
- Synonyms
- 2-METHYLPROPANAL;methylpropanal;ISOBUTYLALDEHYDE;Isobutanal;isobutyral;Isobutyraldehyd;sobutyraldehyde;2-methyl-propana;Propanal,2-methyl-;ISOBUTYRIC ALDEHYDE
- CBNumber:
- CB8350684
- Molecular Formula:
- C4H8O
- Molecular Weight:
- 72.11
- MDL Number:
- MFCD00006980
- MOL File:
- 78-84-2.mol
- MSDS File:
- SDS
| Melting point | -65 °C (lit.) |
|---|---|
| Boiling point | 63 °C (lit.) |
| Density | 0.79 g/mL at 25 °C (lit.) |
| vapor density | 2.5 (vs air) |
| vapor pressure | 66 mm Hg ( 4.4 °C) |
| refractive index |
n |
| FEMA | 2220 | ISOBUTYRALDEHYDE |
| Flash point | −40 °F |
| storage temp. | Store below +30°C. |
| solubility | water: soluble11g/100mL at 20°C(lit.) |
| form | Liquid |
| color | Clear |
| Odor | Pungent. |
| explosive limit | 1.6-11.0%(V) |
| Odor Threshold | 0.00035ppm |
| Odor Type | aldehydic |
| Water Solubility | 75 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| JECFA Number | 252 |
| Merck | 14,5154 |
| BRN | 605330 |
| Stability | Stable. Refrigerate. Highly flammable. Incompatible with strong oxidizing agents, strong bases, strong acids, strong reducing agents. |
| LogP | 0.77 at 25℃ |
| Substances Added to Food (formerly EAFUS) | ISOBUTYRALDEHYDE |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 78-84-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | C42E28168L |
| NIST Chemistry Reference | Propanal, 2-methyl-(78-84-2) |
| EPA Substance Registry System | Isobutyraldehyde (78-84-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xn,Xi | |||||||||
| Risk Statements | 11-22-36 | |||||||||
| Safety Statements | 16-36/37-9-33-29-26 | |||||||||
| RIDADR | UN 2045 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NQ4025000 | |||||||||
| F | 9-13-23 | |||||||||
| Autoignition Temperature | 384 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2912 19 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in rats: 3.7 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Isobutyraldehyde price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W222011 | Isobutyraldehyde natural, 96%, FG | 78-84-2 | 25g | $95.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222011 | Isobutyraldehyde natural, 96%, FG | 78-84-2 | 100g | $193 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222011 | Isobutyraldehyde natural, 96%, FG | 78-84-2 | 1kg | $1250 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222003 | Isobutyraldehyde ≥98%, FG | 78-84-2 | 1kg | $106 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222003 | Isobutyraldehyde ≥98%, FG | 78-84-2 | 4kg | $250 | 2024-03-01 | Buy |
Isobutyraldehyde Chemical Properties,Uses,Production
Description
Isobutyraldehyde, also known as 2-Methylpropanal, is an organic compound belonging to the family of aldehydes, which can be found in alcoholic beverages, tea, breads, cooked pork, spearmint oil as well as fresh fruits, such as apple, banana, cherry, etc. It is manufactured by the hydroformylation of propene, usually obtained as a side-product. It can be applied as a source for producing other chemicals, including isobutyl alcohol, neopentyl glycol as well as isobutanoic acid production and used to produce amino acids such as valine and leucine. Besides, isobutyraldehyde commonly serves as an intermediate in the field of chemical industry for manufacture of pharmaceuticals (such as Vitamin B5), crop protection products, pesticides, synthetic resins, antioxidants, vulcanisation accelerators, textile auxiliaries, perfumery and flavors.
References
https://pubchem.ncbi.nlm.nih.gov/compound/isobutyraldehyde#section=Top
http://chemindustry.ru/Isobutyraldehyde.php
http://product-finder.basf.com/group/corporate/product-finder/en/brand/ISOBUTYRALDEHYDE
https://en.wikipedia.org/wiki/Isobutyraldehyde
Description
Isobutyraldehyde has a characteristic odor. Synthesized via oxidation of isobutyl alcohol with potassium dichromate and concentrated sulfuric acid.
Chemical Properties
Isobutyraldehyde has a characteristic sharp, pungent odor. Industrially, it is mainly hydrogenated to produce isobutanol. The addition reaction of isobutyraldehyde and formaldehyde produces hydroxytrimethylacetaldehyde, which is then hydrogenated to form neopentyl glycol, which is used as a raw material for varnishes, resins, fibers and lubricants.
Physical properties
colourless liquid with an extremely unpleasant smell. Miscible in ethanol, benzene, carbon disulfide, acetone, toluene, chloroform and ether, slightly soluble in water (1:125).
Occurrence
Reported found in apple and currant aromas and in the essential oils from tobacco leaves and tea leaves, also in the essential oils of Pinus jeffreyi Murr. leaves, Citrus aurantium leaves, and Datura stramonium. Reported found in apple, banana, sweet and sour cherry, currants, kohlrabi, carrots, celery, peas, potato, tomato, peppermint, corn mint and spearmint oil, vinegar, wheat and rye breads, cheeses, butter, yogurt, egg, caviar, fatty fish, meats, hop oil, beer, brandy, rum, sherry, cider, whiskies, grape wines, cocoa, coffee, tea, filberts, peanuts, popcorn, oats, soybeans, honey, mushrooms, macadamia nuts, cauliflower, pear and apple brandy, rice, sukiyaki, malt, loquat, clary sage, shrimps, truffle, scallops and squid
Uses
Isobutyraldehyde is used as an intermediate in the preparation of isobutanol, methacrolein, hydroxypivaldehyde and neopentyl glycol. It is actively involved in the Cannizaro reaction. It is also used as an intermediate to prepare pharmaceuticals, agrochemicals, vitamins, antioxidants, rubber accelerators, textile auxiliaries, perfumery and flavors.
Uses
Isobutyraldehyde is used in the synthesisof cellulose esters, resins, and plasticizers;in the preparation of pantothenic acid andvaline; and in flavors.
Uses
In the synthesis of pantothenic acid, valine, leucine, cellulose esters, perfumes, flavors, plasticizers, resins, gasoline additives.
Definition
ChEBI: Isobutyraldehyde is a member of the class of propanals that is propanal substituted by a methyl group at position 2. It has a role as a Saccharomyces cerevisiae metabolite. It is a member of propanals and a 2-methyl-branched fatty aldehyde.
Preparation
By oxidation of isobutyl alcohol with potassium dichromate and concentrated sulfuric acid.
Catalytic synthesis of isobutyraldehyde from methanol and n-propyl alcohol over titanium oxide-supported vanadium oxide catalysts
Aroma threshold values
Detection: 0.4 to 43 ppb
General Description
Isobutyl aldehyde appears as a clear colorless liquid with a pungent odor. Flash point of -40°F. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Oxidizes slowly on exposure to air. Stable (less than 10% decomposition) for four hours when exposed to light and air in a closed system. Stable for two weeks when stored under nitrogen at temperatures up to 77°F. Insoluble in water.
Reactivity Profile
Isobutyraldehyde can react vigorously with reducing agents, with oxidizing agents, strong bases and mineral acids. Can undergo exothermic self-condensation or polymerization reactions that are often catalyzed by acid. Generates flammable and/or toxic gases in combination with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Reacts slowly when exposed to air with air to give peroxides and other products. These reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by their products). The addition of stabilizers (antioxidants) retards autoxidation.
Hazard
Highly flammable, dangerous fire and explosion risk. Irritant to skin and eyes.
Health Hazard
Vapor is irritating to the eyes and mucous membranes.
Health Hazard
Isobutyraldehyde is a moderate skin and eyeirritant; the effect may be slightly greaterthan that of n-butyraldehyde. An amounttotaling 500 mg in 24 hours produced severeskin irritation in rabbits; 100 mg causedmoderate eye irritation.
The toxicity of isobutyraldehyde determinedon test animals was very low. Exposureto 8000 ppm (23,600 mg/m3) for 4 hours waslethal to rats.
LD50 value, oral (rats): 2810 mg/kg.
Fire Hazard
Behavior in Fire: Vapors are heavier than air and may travel considerable distance to a source of ignition and flash back. Fires are difficult to control due to ease of reignition.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Carcinogenicity
Isobutyraldehyde is not mutagenic in various strains of S. typhimurium and is noncarcinogenic in rats and mice.
Purification Methods
Dry isobutyraldehyde with CaSO4 and use it immediately after distillation under nitrogen because of the great difficulty in preventing oxidation. It can be purified through its acid bisulfite derivative. [Beilstein 1 IV 3262.]
Waste Disposal
Isobutyraldehyde is burned in a chemicalincinerator equipped with an afterburner andscrubber.
Isobutyraldehyde Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
View Lastest Price from Isobutyraldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-27 | Isobutyraldehyde
78-84-2
|
US $1.10 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2023-06-30 | Isobutyraldehyde
78-84-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-03-21 | Isobutyraldehyde
78-84-2
|
US $156.00 / kg | 1kg | 99% | 5000 tons | Hebei Duling International Trade Co. LTD |
-

- Isobutyraldehyde
78-84-2
- US $1.10 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- Isobutyraldehyde
78-84-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Isobutyraldehyde
78-84-2
- US $156.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD