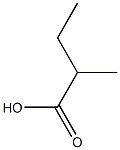
Poly(acrylic acid)
- CAS No.
- 9003-01-4
- Chemical Name:
- Poly(acrylic acid)
- Synonyms
- PAA;9003-1-4;mw;VINYL RESIN;Acrylate polymer;ACRYLIC ACID POLYMER;CARBOPOL 934;CARBOPOL 941;Acrylicresin;KABOMU
- CBNumber:
- CB8708560
- Molecular Formula:
- C5H10O2
- Molecular Weight:
- 102.1317
- MDL Number:
- MFCD00084394
- MOL File:
- 9003-01-4.mol
| Melting point | 95 °C |
|---|---|
| Boiling point | 116 °C |
| Density | 1.2 g/mL at 25 °C |
| vapor pressure | 2.64-3.57hPa at 20-25℃ |
| refractive index |
n |
| Flash point | 100 °C |
| storage temp. | 2-8°C |
| solubility | Swellable in water and glycerin and, after neutralization, in ethanol (95%). Carbomers do not dissolve but merely swell to a remarkable extent, since they are three-dimensionally crosslinked microgels. |
| form | Powder |
| color | White |
| Water Solubility | Soluble in water. |
| Dielectric constant | 2.7 - 4.5(0.0℃) |
| InChIKey | WLAMNBDJUVNPJU-UHFFFAOYSA-N |
| LogP | 0.23-0.27 at 20℃ and pH3.59-3.63 |
| Indirect Additives used in Food Contact Substances | POLY(ACRYLIC ACID) |
| FDA 21 CFR | 701.3 |
| EWG's Food Scores | 1 |
| FDA UNII | 73861X4K5F |
| IARC | 3 (Vol. 19, Sup 7) 1987 |
| EPA Substance Registry System | Polyacrylic acid (9003-01-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H340-H350 | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | C,T,Xi | |||||||||
| Risk Statements | 45-46-34-36/37/38 | |||||||||
| Safety Statements | 53-45-36-27-26 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AT4680000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 39069090 | |||||||||
| Toxicity | LD50 oral in rat: 2500mg/kg | |||||||||
| NFPA 704 |
|
Poly(acrylic acid) price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 181285 | Poly(acrylic acid) average Mv ~450,000 | 9003-01-4 | 5g | $57.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 181285 | Poly(acrylic acid) average Mv ~450,000 | 9003-01-4 | 100g | $207 | 2024-03-01 | Buy |
| Alfa Aesar | 044669 | Poly(acrylic acid), 25 wt% soln. in water | 9003-01-4 | 100g | $51.6 | 2024-03-01 | Buy |
| Alfa Aesar | 044669 | Poly(acrylic acid), 25 wt% soln. in water | 9003-01-4 | 500g | $168 | 2024-03-01 | Buy |
| Sigma-Aldrich | 523925 | Poly(acrylic acid) solution average Mw ~100,000, 35 wt. % in H2O | 9003-01-4 | 100ml | $73.8 | 2024-03-01 | Buy |
Poly(acrylic acid) Chemical Properties,Uses,Production
Description
For a description of unrelated compounds expanded by twocarbon units,Poly acrylic acid (PAA or Carbomer) is generic name for synthetic high molecular weight polymers of acrylic acid. They may be homopolymers of acrylic acid, crosslinked with an allyl ether pentaerythritol, allyl ether of sucrose or allyl ether of propylene. In a water solution at neutral pH, PAA is an anionic polymer, i.e. many of the side chains of PAA will lose their protons and acquire a negative charge. This makes PAAs polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. Dry PAAs are found in the market as white and fluffy powders. Carbomer codes (910, 934, 940, 941 and 934P) are an indication of molecular weight and the specific components of the polymer. For many applications PAAs are used in form of alkali metal or amonium salts e.g. sodium polyacrylate.
Description
Poly (acrylic acid) (PAA) is hygroscopic, brittle and colorless in nature with Tg at nearly 106oC. At temperatures above 200 to 250oC, it loses water and becomes an insoluble crosslinked polymer anhydride. Solubility of dried PAA in water increases with rise in temperatures. Concentrated solutions of PAA in water is thixotropic in nature.
Polyacrylic acid (PAA) is a hydrophilic colloidal solution, similar in properties to water-soluble natural gums. It is a clear, colorless, viscous stable solution. Applications include the modification of aqueous formulations for such end uses as cleaners, binders, adhesives, and emulsion paints. The sodium, potassium, and ammonium salts are effective thickeners and dispersants useful in both natural and synthetic latex systems. PAA in ceramic applications improves dry strength, dispersant action, and improved workability of the clays. PAA is stable to hydrolysis and is not susceptible to bacterial degradation.
Chemical Properties
white powder
Chemical Properties
Carbomers are white-colored, ‘fluffy’, acidic, hygroscopic powders with a characteristic slight odor. A granular carbomer is also available (Carbopol 71G).
Uses
Polyacrylic acid is used in disposable diapers and in ion exchange resins. It is also used to study solute diffusion in polyvinyl alcohol/polyacrylic acid copolymer hydrogel. It is also employed as a thickening, suspending, emulsifying and dispersing agent in pharmaceuticals, cosmetics, adhesives and paints. Further, it is used for the preparation of poly(N-isopropylacrylamide)-block-polyacrylic acid copolymer which responds to both temperature and pH stimuli. In addition to this, it is used in preparing block copolymer of oligo (methyl methacrylate)/PAA for micellar delivery of hydrophobic drugs.
Uses
Applications of PAA may include: · to study solute diffusion in Polyvinyl alcohol/PAA copolymer hydrogel · synthesizing poly(N-isopropylacrylamide)-block-PAA copolymer which responds to both temperature and pH stimuli · in preparing block copolymer of oligo (methyl methacrylate)/PAA for micellar delivery of hydrophobic drugs · as thickening agent for adhesives
Uses
carboxypolymethylene is a binder, film-former and emulsion stabilizer. It can also help increase product viscosity.
Production Methods
Carbomers are synthetic, high-molecular-weight, crosslinked polymers of acrylic acid. These acrylic acid polymers are crosslinked with allyl sucrose or allyl pentaerythritol. The polymerization solvent used previously was benzene; however, some of the newer commercially available grades of carbomer are manufactured using either ethyl acetate or a cyclohexane–ethyl acetate cosolvent mixture. The Carbopol ETD and Carbopol Ultrez polymers are produced in the cosolvent mixture with a proprietary polymerization aid.
Application
Poly acrylic acid and its derivatives are used in disposable diapers,ion exchange resins and adhesives. They are also popular as a thickening, dispersing, suspending and emulsifying agents in pharmaceuticals, cosmetics and paints. PAA inactivates the antiseptic chlorhexidine gluconate.
Definition
ChEBI: An acrylic macromolecule, composed of acrylic acid repeating units.
brand name
Carbopol 934 (Noveon).
General Description
Poly(acrylic acid) solution (PAA) is an anionic polymer that can be synthesized by the free radical polymerization of acrylic acid. It has a swelling nature that tends to absorb and retain the water. Its high ion exchange capacity makes it useful in the formation of membranes.
Pharmaceutical Applications
Carbomers are used in liquid or semisolid pharmaceutical formulations as rheology modifiers. Formulations include creams, gels, lotions and ointments for use in ophthalmic, rectal, topical and vaginal preparations. Carbomer grades with residual benzene content greater than 2 ppm do not meet the specifications of the PhEur 6.4 monograph. However, carbomer having low residuals of other solvents than the ICH-defined ‘Class I OVI solvents’ may be used in Europe. Carbomer having low residuals of ethyl acetate, such as Carbopol 971P NF or Carbopol 974P NF, may be used in oral preparations, in suspensions, capsules or tablets. In tablet formulations, carbomers are used as controlled release agents and/or as binders. In contrast to linear polymers, higher viscosity does not result in slower drug release with carbomers. Lightly crosslinked carbomers (lower viscosity) are generally more efficient in controlling drug release than highly crosslinked carbomers (higher viscosity). In wet granulation processes, water, solvents or their mixtures can be used as the granulating fluid. The tackiness of the wet mass may be reduced by including talc in the formulation or by adding certain cationic species to the granulating fluid. However, the presence of cationic salts may accelerate drug release rates and reduce bioadhesive properties. Carbomer polymers have also been investigated in the preparation of sustained-release matrix beads, as enzyme inhibitors of intestinal proteases in peptide-containing dosage forms, as a bioadhesive for a cervical patch and for intranasally administered microspheres, in magnetic granules for site-specific drug delivery to the esophagus, and in oral mucoadhesive controlled drug delivery systems. Carbomers copolymers are also employed as emulsifying agents in the preparation of oil-in-water emulsions for external administration. Carbomer 951 has been investigated as a viscosity-increasing aid in the preparation of multiple emulsion microspheres. Carbomers are also used in cosmetics. Therapeutically, carbomer formulations have proved efficacious in improving symptoms of moderate-to-severe dry eye syndrome.
Safety
Carbomers are used extensively in nonparenteral products,
particularly topical liquid and semisolid preparations. Grades
polymerized in ethyl acetate may also be used in oral formulations. There is no evidence of systemic absorption of
carbomer polymers following oral administration. Acute oral
toxicity studies in animals indicate that carbomer 934P has a low
oral toxicity, with doses up to 8 g/kg being administered to dogs
without fatalities occurring. Carbomers are generally regarded as
essentially nontoxic and nonirritant materials; there is no
evidence in humans of hypersensitivity reactions to carbomers
used topically.
LD50 (guinea pig, oral): 2.5 g/kg for carbomer 934
LD50 (guinea pig, oral): 2.5 g/kg for carbomer 934P
LD50 (guinea pig, oral): 2.5 g/kg for carbomer 940
LD50 (mouse, IP): 0.04 g/kg for carbomer 934P
LD50 (mouse, IP): 0.04 g/kg for carbomer 940
LD50 (mouse, IV): 0.07 g/kg for carbomer 934P
LD50 (mouse, IV): 0.07 g/kg for carbomer 940
LD50 (mouse, oral): 4.6 g/kg for carbomer 934P
LD50 (mouse, oral): 4.6 g/kg for carbomer 934
LD50 (mouse, oral): 4.6 g/kg for carbomer 940
LD50 (rat, oral): 10.25 g/kg for carbomer 910
LD50 (rat, oral): 2.5 g/kg for carbomer 934P
LD50 (rat, oral): 4.1 g/kg for carbomer 934
LD50 (rat, oral): 2.5 g/kg for carbomer 940
LD50 (rat, oral): > 1g/kg for carbomer 941
No observed adverse effect level (NOAEL) (rat, dog, oral): 1.5 g/kg
for carbomer homopolymer type B.
storage
Carbomers are stable, hygroscopic materials that may be heated at
temperatures below 1048℃ for up to 2 hours without affecting their
thickening efficiency. However, exposure to excessive temperatures
can result in discoloration and reduced stability. Complete
decomposition occurs with heating for 30 minutes at 2608℃. Dry
powder forms of carbomer do not support the growth of molds and
fungi. In contrast, microorganisms grow well in unpreserved
aqueous dispersions, and therefore an antimicrobial preservative
such as 0.1% w/v chlorocresol, 0.18% w/v methylparaben–0.02%
w/v propylparaben, or 0.1% w/v thimerosal should be added. The
addition of certain antimicrobials, such as benzalkonium chloride
or sodium benzoate, in high concentrations (0.1% w/v) can cause
cloudiness and a reduction in viscosity of carbomer dispersions.
Aqueous gels may be sterilized by autoclaving with minimal
changes in viscosity or pH, provided care is taken to exclude oxygen
from the system, or by gamma irradiation, although this technique
may increase the viscosity of the formulation. At room
temperature, carbomer dispersions maintain their viscosity during
storage for prolonged periods. Similarly, dispersion viscosity is
maintained, or only slightly reduced, at elevated storage temperatures
if an antioxidant is included in the formulation or if the
dispersion is stored protected from light. Exposure to light causes
oxidation that is reflected in a decrease in dispersion viscosity.
Stability to light may be improved by the addition of 0.05–0.1%
w/v of a water-soluble UV absorber such as benzophenone-2 or
benzophenone-4 in combination with 0.05–0.1% w/v edetic acid.
Carbomer powder should be stored in an airtight, corrosionresistant
container and protected from moisture. The use of glass,
plastic, or resin-lined containers is recommended for the storage of
formulations containing carbomer.
Current market and forecast
The global demand on acrylic resin approached roughly US $ 14.5 billion in 2011. With an annual growth rate of 4 - 5 % , the acrylic resin market is expected to reach US $ 16.6 billion by 2014 and US$22 billion by 2020. Acrylic resins are used in a wide range of applications for the outstanding chemical characteristics and unique aesthetic properties. Currently, the strongest demand comes from automotive and medical device markets, and paints & coatings, adhesive & sealant and construction & architecture are the major application markets for acrylic resin.
Formulae
Acrylic resin is a general term for any one of the plastics (resin) generated through chemical reaction by applying polymerization initiator and heat to a monomer.
The chemical name for the resin produced from the methyl methacrylate monomer (MMA) is polymethyl methacrylate (PMMA). MMA is a transparent and colorless fluid substance.One of the main characteristic features of PMMA is its high transparency. With its high weather resistance, it has been known to last over 30 years, it does not easily turn yellow or crumble when exposed to sunlight. Polymethyl methacrylate is used not only for transparent windows in aquariums but also for various items such as signboards in places like convenience stores, taillights of automobiles, bathtub liners, sinks, cell phone display screens, backlight optical waveguides for liquid crystal displays (LCD) and so on.
Advantages
The advantages of acrylic resins are :
Better stain protection (wash ability)
Water resistance
Better adhesion
Better blocking ('strap down')
Resist cracking and blistering better
Resistance to alkali cleaners.
Incompatibilities
Carbomers are discolored by resorcinol and are incompatible with
phenol, cationic polymers, strong acids, and high levels of
electrolytes. Certain antimicrobial adjuvants should also be avoided
or used at low levels. Trace levels of iron and other
transition metals can catalytically degrade carbomer dispersions.
Certain amino-functional actives form complexes with carbomer;
often this can be prevented by adjusting the pH of the
dispersion and/or the solubility parameter by using appropriate
alcohols and polyols.
Carbomers also form pH-dependent complexes with certain
polymeric excipients. Adjustment of pH and/or solubility parameter
can also work in this situation.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral suspensions, tablets; ophthalmic, rectal, topical, transdermal preparations; vaginal suppositories). Included in nonparenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Poly(acrylic acid) Preparation Products And Raw materials
Raw materials
1of5
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 476 | 58 |
| Hubei Xindesheng Material Technology Co., Ltd. | +8618971041571 | vickyzhao@whdschem.com | China | 174 | 58 |
| Hengshui Haoye Chemical Co.,Ltd. | +86-2102300 +86-18632882519 | hy@chemcoms.com | China | 256 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 147 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 893 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
Related articles
- Application of Poly(acrylic acid)
- Poly(acrylic acid) (PAA) is a high molecular weight polymer with good water solubility made by polymerisation of acrylic monom....
- Apr 19,2024
- Poly(acrylic acid): Uses, Synthesis and Solubility
- The passage introduces the synthesis, uses and solubility of Poly(acrylic acid).
- Nov 21,2022
- What is Poly(acrylic acid)?
- Poly(acrylic acid) or PAA (CAS number 9003-1-04) is a type of polymer. The monomer of poly(acrylic acid) is Acrylic Acid.
- Jul 23,2020
View Lastest Price from Poly(acrylic acid) manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | carbomer 940
9003-01-4
|
US $20.00 / KG | 100KG | 99% | 1000kg/day | Hebei Yime New Material Technology Co., Ltd. | |
 |
2024-04-24 | acrylic resin
9003-01-4
|
US $8.90 / L | 1L | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2024-04-22 | Carbomer 980/940
9003-01-4
|
US $0.00 / Kg/Bag | 2Kg/Bag | 99% up | 20 tons | Sinoway Industrial co., ltd. |
-

- carbomer 940
9003-01-4
- US $20.00 / KG
- 99%
- Hebei Yime New Material Technology Co., Ltd.
-

- acrylic resin
9003-01-4
- US $8.90 / L
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
-

- Carbomer 980/940
9003-01-4
- US $0.00 / Kg/Bag
- 99% up
- Sinoway Industrial co., ltd.
9003-01-4(Poly(acrylic acid))Related Search:
1of4