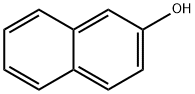
2-Naphthol
- CAS No.
- 135-19-3
- Chemical Name:
- 2-Naphthol
- Synonyms
- naphthalen-2-ol;2-naphthalenol;2-HYDROXYNAPHTHALENE;BETA NAPTHOL;BETA-NAPHTHOL;2-Naphtol;β-Naphthol;B-NAPHTHOL;2-Naftol;BETA NAPHTHO
- CBNumber:
- CB9336283
- Molecular Formula:
- C10H8O
- Molecular Weight:
- 144.17
- MDL Number:
- MFCD00004067
- MOL File:
- 135-19-3.mol
- MSDS File:
- SDS
| Melting point | 120-122 °C(lit.) |
|---|---|
| Boiling point | 285-286 °C(lit.) |
| Density | 1,28 g/cm3 |
| vapor density | 4.97 (vs air) |
| vapor pressure | 10 mm Hg ( 145.5 °C) |
| refractive index | 1.5762 (estimate) |
| Flash point | 153 °C |
| storage temp. | Store below +30°C. |
| solubility | methanol: soluble1g/10 mL, clear, colorless to light yellow |
| pka | 9.51(at 25℃) |
| form | Powder, Crystals or Granules |
| color | White |
| PH Range | Non& uorescence (8.5) to blue & uorescence (9.5) |
| Odor | faint phenol-like odor |
| Water Solubility | 1 g/L (20 ºC) |
| λmax | 226nm, 265nm, 275nm, 286nm, 320nm, 331nm |
| Merck | 14,6384 |
| BRN | 742134 |
| Stability | Stable. Combustible. Dust may form explosive mixture with air. Incompatible with strong oxidizing agents, phenol. |
| Major Application | Display device, semiconductors, photoimaging materials, inks, toner, chalk, security paper, molding materials, tin plating method, rubber, adhesive, leather, detergent, hair dyes, antimitotic drug, anticancer agent, antiinflammatory agent, treatment of acne vulgaris (pimples) and other dermal ailments (rashes, scratches, blemishes, hair loss), disorders |
| InChIKey | JWAZRIHNYRIHIV-UHFFFAOYSA-N |
| LogP | 1.89 at 20℃ |
| Indirect Additives used in Food Contact Substances | BETA-NAPHTHOL |
| FDA 21 CFR | 176.200 |
| CAS DataBase Reference | 135-19-3(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
| FDA UNII | P2Z71CIK5H |
| NIST Chemistry Reference | 2-Naphthalenol(135-19-3) |
| EPA Substance Registry System | 2-Naphthalenol (135-19-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H332-H317-H318-H400 | |||||||||
| Precautionary statements | P273-P280-P301+P312-P302+P352-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N | |||||||||
| Risk Statements | 20/22-50 | |||||||||
| Safety Statements | 24/25-61 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QL2975000 | |||||||||
| F | 8 | |||||||||
| Autoignition Temperature | 430 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29071590 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1960 mg/kg LD50 dermal Rabbit > 10000 mg/kg | |||||||||
| NFPA 704 |
|
2-Naphthol price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22290 | 2-Naphthol for synthesis | 135-19-3 | 250g | $48.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22290 | 2-Naphthol for synthesis | 135-19-3 | 1kg | $76.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 130109 | 2-Naphthol 98% | 135-19-3 | 250g | $53.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 130109 | 2-Naphthol 98% | 135-19-3 | 1kg | $72.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 70450 | 2-Naphthol fluorescence indicator, ≥99.0% | 135-19-3 | 500g | $161 | 2022-05-15 | Buy |
2-Naphthol Chemical Properties,Uses,Production
Chemical Properties
2-Naphthol is a white, crystalline solid. Slight phenolic odor. Darkens in air and on exposure to light. 2-Naphthol [135-19-3] b-naphthol, 2- naphthalenol, 2-hydroxynaphthalene, C10H8O, Mr 144.16, forms colorless plates upon sublimation, which darken on exposure to air or light. Unlike 1-naphthol it is nonvolatile in steam. 2-Naphthol is reduced with sodium or with hydrogen in the presence of a catalyst to give mainly 1,2,3,4-tetrahydro-2-naphthol (unlike 1- naphthol which gives the arylphenol under the same conditions). Oxidation with ferric chloride forms 2,20 -dihydroxy-1,10 -binaphthyl, with any blue coloration indicating the presence of 1- naphthol. Air oxidation at 300℃ with a vanadium pentoxide catalyst also yields 1,10 -bi-2-naphthol, which dehydrates at higher temperature to the oxide and finally decomposes to give benzoic acid. Treatment with sulfuryl chloride in carbon disulfide or with NaOCl – NaOH leads to 1- chloro-2-naphthol, whereas chlorine in sodium carbonate gives 8-chloro-2-naphthol ( plus 1, 10 - bi-2-naphthol). Reaction with phosphorus pentachloride at 150℃ gives 2-chloronaphthalene or at lower temperature, tri-2-naphthyl phosphate (mp 111℃), which can be isolated after treatment of the reaction mixture with alkali. Reaction with one equivalent of bromine in acetic acid gives 1-bromo-2-naphthol, whereas excess bromine yields 1,6-dibromo-2-naphthol. The latter is readily debrominated in dilute mineral acid to yield 6-bromo-2-naphthol. Excess bromine reacts with 2-naphthol at 100℃ to form 1,5,6- tribromo- and 1,3,5,6-tetrabromo-2-naphthol.
Physical properties
White glossy flakes or white powder. Insoluble in water, soluble in ethanol, ether, chloroform, glycerol and alkali solution.
Uses
Has been used as antiseptic, anthelmintic and counter-irritant in alopecia.
Uses
2-Naphthol is used in the manufacture of dyes, perfumes, and medicinal organics, and in the production of antioxidants for synthetic rubber.
Preparation
2-Naphthol is produced by
caustic fusion of naphthalene-2-sulfonic acid. Typically, the sodium salt of the
sulfonic acid is added gradually to 50 % sodium
hydroxide liquor at 300℃; the melt is then
heated further at 320℃ in a gas-fired iron vessel
with vigorous agitation. After completion of the
reaction, the melt is run into excess water, possibly including filtrate from the previous batch at a
proven tolerable level, and the naphtholate solution is neutralized to pH 8 with dilute sulfuric
acid. If the temperature is maintained at >100℃ during neutralization, the crude product comes
out of solution as an oil, which is separated,
washed with hot water, and distilled under vacuum to give pure 2-naphthol. The molten material
is processed through a flaker to give the final
product for packaging. The fusion yield is about
80 % of the theoretical value, resulting in an
overall yield of 70 % based on naphthalene.
Typical specifications for 2-naphthol are clear
solution in dilute caustic soda, mp 120.5℃,
and 1-naphthol content<0.3 %.
The newer method of manufacture is economically and environmentally favored in the United States, because despite requiring three stages, it is more amenable to
continuous operation with recycle streams. The
alkylation and isomerization are carried out up to
240℃ with a phosphoric acid catalyst.
Final catalytic oxidation at 90 – 110℃ gives
the hydroperoxide, which is cleaved with dilute
sulfuric acid to give 2-naphthol in high overall
yield in spite of modest oxidation conversion.
Definition
ChEBI: 2-naphthol is a naphthol carrying a hydroxy group at position 2. It has a role as an antinematodal drug, a genotoxin, a human xenobiotic metabolite, a mouse metabolite, a human urinary metabolite and a radical scavenger.
Application
It is used to produce Tobias acid, J acid, 2-hydroxy-3-naphthoic acid and azo dyes, and it is the raw material for rubber antioxidants, mineral dressing agents, fungicides, antiseptics, preservatives, etc.
As feed preservative. In China, it can be used for citrus preservation, the maximum dosage amount is 0.1 g/kg and the residue amount should be no more than 70 mg/kg.
2-Naphthol, also called ?-naphthol, 2-naphthalenol, is the intermediate for plant growth regulator, 2-naphthoxyacetic acid.
As analytic agent, absorbent of ethylene and carbon monoxide, and fluorescence indicator.
Important organic raw material and dye intermediate, used to produce Tobias acid, butyric acid, β-hydroxynaphthoic acid and used to produce N-phenyl-2-naphthylamine, Diafen NN and other antioxidant, organic pigments, and fungicides.
For the detection of bromine, chlorine, chlorate, niobium, copper, nitrite, and potassium. Substrate for fluorometric assay of phenol sulfotransferase. Acid and alkali indicator, dyes, organic synthesis, qualitative determination of allyl alcohol, methanol, chloroform, etc. Absorbent of carbon monoxide, ethanol, and fluorescence indicator. Determination of carbon monoxide, copper, nitrite, and potassium. Ethylene absorbent.
Reactions
2-Naphthol is naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols.2-Naphthol converts to 2-naphthalenethiol by reaction with dimethylthiocarbamoyl chloride via the Newman–Kwart rearrangement.
Synthesis Reference(s)
Journal of the American Chemical Society, 72, p. 4884, 1950 DOI: 10.1021/ja01167a009
Synthesis, p. 437, 1985 DOI: 10.1055/s-1985-31235
General Description
2-Naphthol (2OH) is a hydroxyarene molecule, which when electronically excited forms strong acid. Excited 2OH dissociates only in water. It has a slight phenolic odor. It is incompatible with strong oxidizing agents, strong bases, acid chlorides and acid anhydrides. It is one of the most commonly used fluorescence dye.
Hazard
See α-naphthol
Health Hazard
Although the toxicity of 2-naphthol is of low order in test animals, ingestion of large amounts may result in nausea, vomiting, diarrhea, abdominal pain, convulsions, and hemolytic anemia. Death may result from respiratory failure. The oral LD50 value in rats is in the range 2000 mg/kg. 2-Naphthol is slightly more toxic than 1-naphthol [9015-3], the oral LD50 value of which is in the range 2500 mg/kg.
Skin contact can produce peeling of the skin and pigmentation.
Fire Hazard
Noncombustible solid.
Safety Profile
Poison by ingestion, inhalation, and subcutaneous routes. Mutation data reported. A skin and eye irritant. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical. Incompatible with antipyrine, camphor, phenol, ferric salts, menthol, potassium permanganate and other oxidzing materials, urethane.
Potential Exposure
A potential danger to those involved in rubber antioxidant production, synthesis of dyes; leather processing; fungicides, pharmaceuticals, and perfumes. Used as an antioxidant for fats, oils; as an antiseptic; in insecticides.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise 2-naphthol from aqueous 25% EtOH (charcoal), H2O, *benzene, toluene or CCl4. Alternatively, extract it repeatedly with small amounts of EtOH, followed by dissolution in a minimum volume of EtOH and precipitation with distilled water, then drying over P2O5 under vacuum. It has also been dissolved in aqueous NaOH and precipitated by adding acid (repeat several times), then precipitated from *benzene by addition of heptane. Final purification can be by zone melting or sublimation in vacuo. The 4-nitrobenzoate has m 104o (from EtOH). [Bardez et al. J Phys Chem 89 5031 1985, Kikuchi et al. J Phys Chem 91 574 1987, Beilstein 6 IV 4253.]
Properties and Applications
white crystalline, with phenol smell. Industrial goods for hoar chip or powder. Melting point 121 ~ 123 ℃, 285 ℃ ~ 286 boiling point, flash point 161 ℃. This product soluble in water, ethanol, ethyl ether, chloroform, glycerin and alkali solution, can the sublimation, with steam evaporate out; Long storage or light color in turn dark, can happen oxidation, reduction, nitrification and nitrosation reaction, and products, light and heat, halogenating role, etc.
Incompatibilities
Dust or powder may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, iron salts; 2,3-dimethyl-1- phenyl-3-pyrazolin-5-one (antipyrine); camphor, phenol, menthol, urethane.
Waste Disposal
Mix with flammable solvent and atomize into an incinerator.
2-Naphthol Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 990 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 16805 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 958 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 | sales@richfortunechem.com | China | 303 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
Related articles
- 2-Naphthol: Polarity; Solubility and Uses
- 2-Naphthol is a polar molecule. Since naphthol contains hydroxyl groups, it is a very polar molecule, and the oxygen atom attr....
- Jan 4,2024
- 2-Naphthol: properties and applications in synthesis of heterocyclic compounds and health risks
- 2-Naphthol is valuable in organic synthesis but poses health risks, especially for children, due to its link to oxidative stre....
- Dec 15,2023
- 2-Naphthol:Production,Human Health,Environment
- 2-Naphthol is produced by sulfonation of naphthalene to 2-naphthalene sulfonic acid, and reaction with caustic soda solution ....
- Feb 22,2023
View Lastest Price from 2-Naphthol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-30 | 2-Naphthol
135-19-3
|
US $30.00 / kg | 3kg | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-04-30 | 2-Naphthol
135-19-3
|
US $2.00 / KG | 1KG | 99% | 1000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-30 | 2-Naphthol
135-19-3
|
US $50.00-30.00 / kg | 1kg | 99% | 20Tons | Hebei Dangtong Import and export Co LTD |
-

- 2-Naphthol
135-19-3
- US $30.00 / kg
- 98%
- hebei hongtan Biotechnology Co., Ltd
-

- 2-Naphthol
135-19-3
- US $2.00 / KG
- 99%
- Jinan Finer Chemical Co., Ltd
-

- 2-Naphthol
135-19-3
- US $50.00-30.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD