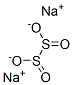히드로아황산 나트륨
|
|
히드로아황산 나트륨 속성
- 녹는점
- 300 °C
- 끓는 점
- 1390°C
- 밀도
- 2.13
- 인화점
- >100°C
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 250g/L(20°C)
- 물리적 상태
- 분말/고체
- 색상
- 하얀색
- 냄새
- 이산화황 냄새가 없거나 약간 있음
- 수소이온지수(pH)
- 5.5-8.5 (50g/l, H2O, 20℃)
- 수용성
- 250 g/L (20 ºC)
- 감도
- Moisture Sensitive
- Merck
- 14,8626
- 안정성
- 안정적이지만 공기에 민감합니다. 강산, 강산화제, 물, 습기와 호환되지 않습니다.
- LogP
- -2.756 (est)
- CAS 데이터베이스
- 7775-14-6(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 7-22-31 | ||
| 안전지침서 | 26-28-43-7/8-43E-28A | ||
| 유엔번호(UN No.) | UN 1384 4.2/PG 2 | ||
| WGK 독일 | 1 | ||
| F 고인화성물질 | 1-10 | ||
| 자연 발화 온도 | >200 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 4.2 | ||
| 포장분류 | II | ||
| HS 번호 | 28311010 | ||
| 유해 물질 데이터 | 7775-14-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2500 mg/kg | ||
| 기존화학 물질 | KE-31508 |
히드로아황산 나트륨 C화학적 특성, 용도, 생산
순도시험
(1) 용상 : 포르말린 10mL에 물 10mL를 가하여 수산화나트륨시액으로 중화하고 그 액 10mL에 이 품목 0.5g을 가하여 녹이고 5분간 방치할 때, 그 탁도는 미탁 이하이어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
(4) 아연 : 위 (3)의 A액 5mL에 암모니아시액 0.1mL를 가하여 여과하고 여액에 물을 가하여 20mL로 하고 묽은염산 5mL 및 페로시안화칼륨용액 0.1mL를 가하여 15분간 방치할 때, 그 탁도는 아연표준용액 8mL에 물을 가하여 20mL로 하고 묽은염산 5mL 및 페로시안화칼륨시액 0.1mL를 가하여 15분간 방치한 액의 탁도 이하이어야 한다.
(5) 에틸렌디아민사초산이나트륨 : 이 품목 0.5g을 물 5mL에 녹이고 0.5% 크롬산칼륨용액 2mL 및 아비산시액 2mL를 가하여 수욕중에서 2분간 가열할 때, 자색을 나타내어서는 아니 된다.
(6) 개미산 : 이 품목의 수용액(1→1,000) 10mL에 염산(1→2) 5mL를 가하고 다음에 마그네슘가루 0.3g을 소량씩 가하여 거품이 거의 발생하지 않으면 시계접시를 덮고 2시간 방치한다. 그 중 1mL를 취해 황산 2mL 및 크로모트로프산시액 0.5mL를 가하고 수욕중에서 10분간 가열할 때, 그 액의 색은 따로 검체대신 묽은포름알데히드표준용액 1mL를 취해 검체와 같이 조작하여 얻은 액의 색보다 진하여서는 아니 된다.
확인시험
(1) 이 품목의 수용액(1→100) 10mL에 황산동시액 1mL를 가하면 시액의 색을 나타낸다.
(2) 이 품목의 수용액(1→100) 10mL에 과망간산칼륨시액 1mL를 가하면 시액의 색은 곧 없어진다.
(3) 이 품목은 확인시험법 중 나트륨염의 (가) 및 (나)의 반응을 나타낸다.
정량법
포르말린 10mL에 물 10mL를 가하여 수산화나트륨시액으로 중화하고 이에 이 품목 약 2g을 정밀히 달아 가하고, 물을 가하여 녹여 500mL로 한다. 그 중 25mL에 염산(1→10)을 가하여 pH 1.1~1.5로 한 다음 0.1N 요오드용액으로 적정한다(지시약 : 전분시액).
0.1N 요오드용액 1mL = 4.353mg Na2S2O4
화학적 성질
White solid용도
Sodium dithionite is a reducing agent for vat dyes,reducing hair bleaching agents,vat dyes printing auxiliaries, silk scouring and bleaching agents, coloring agents and objects stripping vat cleaning agent, particularly in dyeing with indigo and vat dyes; bleaching soaps, straw; removing dyes from dyed fabrics.정의
ChEBI: An inorganic sodium salt that is the disodium salt of dithionous acid.제조 방법
Sodium dithionite is today produced mainly from sodium formate and sulfur dioxide. Zinc dithionite, prepared on-site from sulfur dioxide and zinc dust, was formerly more important than the sodium salt. The heavy metal zinc ended up in the effluent, which caused environmental problems. Today in pulp mills on-site production of zinc dithionite is no longer practiced. The number of plants using sodium amalgam and sulfur dioxide for dithionite production is decreasing with the number of amalgam cells in electrolysis. On-site generation of dithionite from alkaline sodium borohydride solution, sodium bisulfite, and sulfur dioxide is used by some mills.Sodium dithionite is available as crystalline powder (&90% Na2S2O4) or as a refrigerated 150 g/L solution stabilized with alkali. The white crystals can decompose on heating. Low alkalinity, pH8 to 13, stabilizes dithionite solution at low temperature.
These solutions should be kept well below 10 °C with the exclusion of air. In presence of air, oxidation rapidly yields sulfate and sulfite.
The powder product prepared via the amalgam route dissolves into an alkaline solution as it contains sodium carbonate and sulfite. This can trigger the precipitation of calcium carbonate (water hardness) in the solution tank. Therefore, it is recommended to add small amounts of chelants during the dissolution step. (Chelants are not required to stabilize the bleaching reaction.) Sodium dithionite prepared by the formate route dissolves into a slightly acidic solution. Dithionite powder can ignite if exposed to high humidity or elevated temperature.
생산 방법
An alternative route for dithionite production is the reduction of sodium bisulfite with sodium borohydride. Sodium borohydride is obtained by reacting boron trimethyl ester, B(OCH3)3, with sodium hydride, NaH. The resulting product is hydrolyzed with water, and methanol is evaporated. An alkaline, aqueous solution is obtained, containing about 12% NaBH4 and 40% NaOH. This solution is commercially available. Reaction to dithionite is made on-site by adding sulfur dioxide and some additional caustic soda. Storages, handling and mixing of sulfur dioxide and of the Borol® liquid are not everywhere cost competitive.NaBH4+8NaOH+8SO2 → 4Na2S2O4+NaBO2+6H2O.
일반 설명
Sodium dithionite is a whitish to light yellow crystalline solid having a sulfur dioxide-like odor. Sodium dithionite spontaneously heats on contact with air and moisture. This heat may be sufficient to ignite surrounding combustible materials. Sodium dithionite is soluble in water. Under prolonged exposure to fire or intense heat containers of Sodium dithionite may violently rupture. Sodium dithionite is used in dyeing and to bleach paper pulp.반응 프로필
Inorganic reducing agents, such as Sodium dithionite, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Their reactions with oxidizing agents may be violent. Sulfites and hydrosulfites (dithionites) can react explosively with strong oxidizing agents (sodium chlorite). Sulfites generate gaseous sulfur dioxide in contact with oxidizing acids and nonoxidizing acids.위험도
Fire risk in contact with moisture. To extin- guish fires, flood the reacting mass with water.건강위험
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.화재위험
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.Safety Profile
Toxic and an irritant. An allergen. Flammable when exposed to heat or flame. Ignites on contact with water or sodium chlorite. To extinpsh fires, flood the reacting mass with water. Decomposes violently when heated to 19OOC and emits toxic fumes of SOx and NazO저장
Sodium dithionite crystals are available in steel containers (1 or 2 tons) or in steel drums (200 kg). Because of the danger of spontaneous ignition in humid air, sodium dithionite must be stored under dry and cool conditions. The sites for the preparation of dithionite solutions must permit handling without risks, high humidity should be avoided and remote fire control should be available.Commercial dithionite products are classified as self-igniting hazardous goods (Class 4.2, UN 1384). Local rules for transportation and storage must be obeyed.
히드로아황산 나트륨 준비 용품 및 원자재
원자재
준비 용품
히드로아황산 나트륨 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |