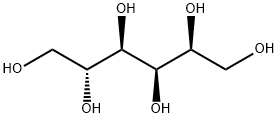
D-솔비톨
|
|
D-솔비톨 속성
- 녹는점
- 98-100 °C (lit.)
- 알파
- 4 º (per eur. pharm.)
- 끓는 점
- bp760 105°
- 밀도
- 1.28 g/mL at 25 °C
- 증기 밀도
- <1 (vs air)
- 증기압
- <0.1 mm Hg ( 25 °C)
- 굴절률
- n
20/D 1.46
- FEMA
- 3029 | D-SORBITOL
- 인화점
- >100°C
- 저장 조건
- room temp
- 용해도
- 물에 매우 잘 녹고 에탄올에 약간 녹는다
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- pKa (17.5°): 13.6
- Specific Gravity
- 1.28
- 색상
- 하얀색
- 수소이온지수(pH)
- 5.0-7.0 (25℃, 1M in H2O)
- 냄새
- 냄새 없는
- pH 범위
- 5 - 7 at 182 g/l at 25 °C
- optical activity
- [α]20/D 1.5±0.3°, c = 10% in H2O
- ?? ??
- 캐러멜 같은
- 수용성
- 녹는
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.04
λ: 280 nm Amax: 0.045
- Merck
- 14,8725
- BRN
- 1721899
- Dielectric constant
- 33.5(27℃)
- 안정성
- 안정적인. 강한 산화제를 피하십시오. 습기로부터 보호하십시오.
- InChIKey
- FBPFZTCFMRRESA-JGWLITMVSA-N
- LogP
- -4.67
- CAS 데이터베이스
- 50-70-4(CAS DataBase Reference)
- NIST
- Sorbitol(50-70-4)
- 위험 및 안전 성명
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 8-36-26-24/25 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | LZ4290000 | ||
| F 고인화성물질 | 3 | ||
| 자연 발화 온도 | 420 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29054491 | ||
| 유해 물질 데이터 | 50-70-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 15900 mg/kg | ||
| 기존화학 물질 | KE-31708 |
D-솔비톨 C화학적 특성, 용도, 생산
물성
글리세린보다 보습력은 적지만, 실온에 고체라는 장점이 있다. 어묵에도 거의 대부분 들어간다. 게맛살에도 어육의 냉동과정에서 풍미를 지키기 위해 D-소르비톨이 들어간다. 감미도는 0.6 정도로, 소르비톨 세 숟갈은 설탕 두 숟갈과 같은 단맛이라 보면 된다.개요
당알코올의 일종. 글리시톨, 솔비톨이라고도 한다. 사과, 복숭아에 함유되어 있다. 겉보기엔 설탕과 매우 흡사하며(당알코올이 다 그렇지만) 포도당을 수소로 환원시켜서 얻을 수 있다. 비타민C의 중간 물질이기도 하다. 또 다른 당알코올인 만니톨과 이성질체 관계다.용도
당뇨병 환자를 위한 감미료로 쓰이는 경우가 많다. 이외에도 글리세린처럼 보습 효과가 있어 화장품, 비누의 첨가물로도 쓰이며 자일리톨처럼 단맛을 내면서도 세균이 분해를 할 수 없어 치약에도 쓰인다.순도시험
(1) 비중 : 이 품목의 비중은 1.285 이상이어야 한다.
(2) 유리산 : 이 품목 5g을 새로 끓여서 식힌 물 50mL에 녹이고 페놀프탈레인시액 1방울 및 0.01N 수산화나트륨용액 0.5mL를 가하여 흔들어 섞어줄 때, 30초 이상 지속하는 홍색을 나타내어야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(5) 니켈 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(6) 당류 : 이 품목 10g을 물 25mL에 녹이고 묽은염산 8mL를 가하여 환류냉각기를 연결하여 수욕 중에서 3시간 가열하고 식힌 다음 메틸오렌지시액을 지시약으로 하여 수산화나트륨시액으로 중화한다. 다음에 물을 가하여 100mL로 하고 그 중 10mL에 물 10mL 및 펠링시액 40mL를 가하여 3분간 조용히 끓인 다음 방치하여 아산화동을 침전시킨다. 다음에 상징액을 유리여과기로 여과하여 플라스크내의 침전은 씻은 액이 알칼리성을 나타내지 않을 때까지 온탕으로 씻고 씻은 액은 유리여과기로 여과한다. 플라스크내의 침전에 황산제이철시액 20mL를 가하여 녹이고 이를 위의 유리여과기로 여과하여 물로 씻고 그 씻은 액을 합쳐 80℃로 가열하고 0.1N 과망간산칼륨용액 20mL를 가할 때, 시액의 색이 곧 없어져서는 아니 된다.
(7) 환원당 : 이 품목 1g을 물 25mL에 녹이고 펠링시액 40mL를 가하여 3분간 조용히 끓인 다음 이하 순도시험 (6)당류에 따라 시험한다. 다만, 0.1N 과망간산칼륨용액은 2mL를 사용한다.
순도시험
(1) 유리산 : 이 품목 5g을 새로 끓여서 식힌 물 50mL에 녹이고 페놀프탈레인시액 1방울 및 0.01N 수산화나트륨용액 0.5mL를 가하여 흔들어 섞어줄 때, 30초이상 지속하는 홍색을 나타내어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(4) 니켈 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(5) 염화물 : 이 품목 10g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 1.5mL에 대응하는 양 이하이어야 한다(0.005% 이하).
(6) 황산염 : 이 품목 10g을 취하여 황산염시험법에 따라 시험할 때, 그 양은 0.01N 황산 2.0mL에 대응하는 양 이하이어야 한다(0.01% 이하).
(7) 당류 : 이 품목 10g을 물 25mL에 녹이고 묽은염산 8mL를 가하여 환류냉각기를 연결하여 수욕 중에서 3시간 가열하고 식힌 다음 메틸오렌지시액을 지시약으로 하여 수산화나트륨시액으로 중화한다. 다음에 물을 가하여 100mL로 하고 그 중 10mL에 물 10mL 및 펠링시액 40mL를 가하여 3분간 조용히 끓인 다음 방치하여 아산화동을 침전시킨다. 다음에 상징액을 유리여과기로 여과하여 플라스크내의 침전은 씻은 액이 알칼리성을 나타내지 않을 때까지 온탕으로 씻고 씻은 액은 유리여과기로 여과한다. 플라스크내의 침전에 황산제이철시액 20mL를 가하여 녹이고 이를 위의 유리여과기로 여과하여 물로 씻고 그 씻은 액을 합쳐 80℃로 가열하고 0.1N 과망간산칼륨용액 20mL를 가할 때, 시액의 색이 곧 없어져서는 아니 된다.
(8) 환원당 : 이 품목 1g을 물 25mL에 녹이고 펠링시액 40mL를 가하여 3분간 조용히 끓인 다음 이하 순도시험 (7)에 따라 시험한다. 다만, 0.1N 과망간산칼륨용액은 2mL를 사용한다.
확인시험
(1) 액성 : 이 품목의 수용액(7→10) 1mL에 황산제일철시액 2mL 및 수산화나트륨용액(1→5) 1mL를 가하면 액은 청록색을 나타내며 탁하게 되지 아니한다.
(2) 이 품목의 수용액(1→100) 1mL에 새로 만든 카테콜용액(1→10) 1mL를 가하여 잘 흔들어 섞은 다음 황산 2mL를 가하여 흔들어 섞으면 곧 적색을 나타낸다.
확인시험
「D-소비톨」의 확인시험에 따라 시험한다.
정량법
이 품목 약 1g을 정밀히 달아 물에 녹여 500mL로 하여 「D-소비톨」의 정량법에 따라 정량한다.
정량법
이 품목을 건조한 다음 약 1g을 정밀히 달아 물에 녹여 500mL로 하고 그 중 10mL에 0.3% 과요오드산칼륨용액 50mL와 황산 1mL를 가한 다음 수욕상에서 15분간 가열한다. 식힌 다음 요오드칼륨 2.5g을 가하고 잘 흔들어 섞은 다음 차고 어두운 곳에 5분간 방치하고 유리된 요오드를 0.1N 치오황산나트륨용액으로 적정한다(지시약 : 전분시액). 따로 같은 방법으로 공시험을 한다.
0.1N 치오황산나트륨용액 1mL = 1.822mg C6H11O6
강열잔류물
이 품목 5g에 황산 2~3방울을 가하여 조용히 가열하여 끓이고 점화하여 연소시키고 식힌 다음 잔류물에 대하여 강열잔류물시험법에 따라 시험할 때, 그 양은 1mg 이하이어야 한다.
강열잔류물
이 품목 약 5g을 정밀히 달아 강열잔류물시험법에 따라 시험할 때, 그 양은 0.02% 이하이어야 한다.
화학적 성질
Sorbitol is D-glucitol. It is a hexahydric alcohol related to mannose and is isomeric with mannitol.Sorbitol occurs as an odorless, white or almost colorless, crystalline, hygroscopic powder. Four crystalline polymorphs and one amorphous form of sorbitol have been identified that have slightly different physical properties, e.g. melting point. Sorbitol is available in a wide range of grades and polymorphic forms, such as granules, flakes, or pellets that tend to cake less than the powdered form and have more desirable compression characteristics. Sorbitol has a pleasant, cooling, sweet taste and has approximately 50–60% of the sweetness of sucrose.
출처
Sorbitol is one of the most widely found sugar alcohols in nature with relatively high concentrations occurring in apples, pears, plums, peaches and apricots. Also reported found in several varieties of berries, seaweed and algae.용도
Sorbitol is a humectant that is a polyol (polyhydric alcohol) produced by hydrogenation of glucose with good solubility in water and poor solubility in oil. It is approximately 60% as sweet as sugar, and has a caloric value of 2.6 kcal/g. It is highly hygroscopic and has a pleasant, sweet taste. It maintains moistness in shredded coconut, pet foods, and candy. In sugarless frozen desserts, it depresses the freezing point, adds solids, and contributes some sweetness. It is used in low-calorie beverages to provide body and taste. It is used in dietary foods such as sugarless candy, chewing gum, and ice cream. It is also used as a crystallization modifier in soft sugar-based confections.정의
ChEBI: The D-enantiomer of glucitol (also known as D-sorbitol).제조 방법
Sorbitol is manufactured by hydrogenation of glucose with hydrogen and active nickel catalyst. It is commercially available as 70% syrup or as a pure white powder.생산 방법
Sorbitol occurs naturally in the ripe berries of many trees and plants. It was first isolated in 1872 from the berries of the Mountain Ash (Sorbus americana).Industrially, sorbitol is prepared by high-pressure hydrogenation with a copper–chromium or nickel catalyst, or by electrolytic reduction of glucose and corn syrup. If cane or beet sugars are used as a source, the disaccharide is hydrolyzed to dextrose and fructose prior to hydrogenation.
일반 설명
Odorless colorless solid. Sinks and mixes with water.공기와 물의 반응
Water soluble.반응 프로필
D-Sorbitol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.건강위험
Hot liquid will burn skin.Pharmaceutical Applications
Sorbitol is widely used as an excipient in pharmaceutical formulations. It is also used extensively in cosmetics and food products.Sorbitol is used as a diluent in tablet formulations prepared by either wet granulation or direct compression. It is particularly useful in chewable tablets owing to its pleasant, sweet taste and cooling sensation. In capsule formulations it is used as a plasticizer for gelatin. Sorbitol has been used as a plasticizer in film formulations.
In liquid preparations sorbitol is used as a vehicle in sugar-free formulations and as a stabilizer for drug, vitamin, and antacid suspensions. Furthermore, sorbitol is used as an excipient in liquid parenteral biologic formulations to provide effective protein stabilization in the liquid state. It has also been shown to be a suitable carrier to enhance the in vitro dissolution rate of indometacin. In syrups it is effective in preventing crystallization around the cap of bottles. Sorbitol is additionally used in injectable and topical preparations, and therapeutically as an osmotic laxative.
Sorbitol may also be used analytically as a marker for assessing liver blood flow.
Safety Profile
Mildly toxic by ingestion. Human systemic effects by ingestion: hypermotility and diarrhea. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.Safety
Sorbitol is widely used in a number of pharmaceutical products and occurs naturally in many edible fruits and berries. It is absorbed more slowly from the gastrointestinal tract than sucrose and is metabolized in the liver to fructose and glucose. Its caloric value is approximately 16.7 J/g (4 cal/g). Sorbitol is better tolerated by diabetics than sucrose and is widely used in many sugar-free liquid vehicles. However, it is not considered to be unconditionally safe for diabetics.Reports of adverse reactions to sorbitol are largely due to its action as an osmotic laxative when ingested orally,(17–19) which may be exploited therapeutically. Ingestion of large quantities of sorbitol (>20 g/day in adults) should therefore be avoided. Sorbitol is not readily fermented by oral microorganisms and has little effect on dental plaque pH; hence, it is generally considered to be noncariogenic.
Sorbitol is generally considered to be more irritating than mannitol.
LD50 (mouse, IV): 9.48 g/kg
LD50 (mouse, oral): 17.8 g/kg
LD50 (rat, IV): 7.1 g/kg
LD50 (rat, SC): 29.6 g/kg
저장
Sorbitol is chemically relatively inert and is compatible with most excipients. It is stable in air in the absence of catalysts and in cold, dilute acids and alkalis. Sorbitol does not darken or decompose at elevated temperatures or in the presence of amines. It is nonflammable, noncorrosive, and nonvolatile.Although sorbitol is resistant to fermentation by many microorganisms, a preservative should be added to sorbitol solutions. Solutions may be stored in glass, plastic, aluminum, and stainless steel containers. Solutions for injection may be sterilized by autoclaving.
The bulk material is hygroscopic and should be stored in an airtight container in a cool, dry place.
Purification Methods
Crystallise D(-)-sorbitol (as hemihydrate) several times from EtOH/water (1:1), then dry it by fusing and storing over anhydrous MgSO4. [Koch et al. J Am Chem Soc 75 953 1953, Beilstein 1 IV 2839.]비 호환성
Sorbitol will form water-soluble chelates with many divalent and trivalent metal ions in strongly acidic and alkaline conditions. Addition of liquid polyethylene glycols to sorbitol solution, with vigorous agitation, produces a waxy, water-soluble gel with a melting point of 35–40℃. Sorbitol solutions also react with iron oxide to become discolored.Sorbitol increases the degradation rate of penicillins in neutral and aqueous solutions.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (intra-articular and IM injections; nasal; oral capsules, solutions, suspensions, syrups and tablets; rectal, topical, and vaginal preparations). Included in parenteral and nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.D-솔비톨 준비 용품 및 원자재
원자재
준비 용품
D-솔비톨 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 |
hjt@hong-jin.com | China | 228 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Guangzhou Tosun Pharmaceutical Limited | +8618922120635 |
sales@toref-standards.com | China | 1000 | 58 |
| Across Biotech Jinan Co LTD | +8613031735486 |
frank@acrossbiotech.com | China | 105 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 |
admin01@hsnm.com.cn | China | 2118 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 |
admin@juchuangchem.com | China | 387 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 893 | 58 |