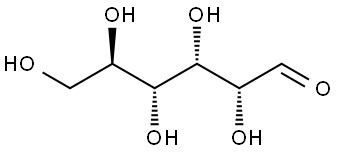
말토덱스트린
|
|
말토덱스트린 속성
- 녹는점
- 150-152 °C(lit.)
- 알파
- 52.75 º (c=10, H2O, NH4OH 25 ºC)
- 끓는 점
- 232.96°C (rough estimate)
- 밀도
- 1.5440
- 굴절률
- 53 ° (C=10, H2O)
- 저장 조건
- room temp
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 산도 계수 (pKa)
- pKa 12.43(H2O,t = 18,)(Approximate)
- 물리적 상태
- 결정성 분말
- 색상
- 하얀색
- 수소이온지수(pH)
- 5.0-7.0 (25℃, 1M in H2O)
- 냄새
- 냄새 없는
- pH 범위
- 5.9
- optical activity
- [α]25/D +52.5 to +53.0°(lit.)
- 수용성
- 녹는
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.02
- Merck
- 14,4459
- BRN
- 1281608
- 안정성
- 안정적인. 피해야 할 물질에는 강한 산화제가 포함됩니다. 타기 쉬운.
- InChIKey
- WQZGKKKJIJFFOK-DVKNGEFBSA-N
- LogP
- -2.490 (est)
- CAS 데이터베이스
- 50-99-7(CAS DataBase Reference)
- NIST
- Glucose(50-99-7)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-63-62-46-36/38-21 | ||
| 안전지침서 | 26-36/37-24/25-53-25 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | LZ6600000 | ||
| F 고인화성물질 | 3 | ||
| 자연 발화 온도 | 500 °C | ||
| TSCA | Yes | ||
| HS 번호 | 17023051 | ||
| 유해 물질 데이터 | 50-99-7(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 25800 mg/kg | ||
| 기존화학 물질 | KE-17727 |
말토덱스트린 C화학적 특성, 용도, 생산
개요
D(+)-Glucose is one of the most important biological compounds found in nature. It is a main product in photosynthesis and is oxidized in cellular respiration. D(+)-Glucose polymerizes to form several important classes of biomolecules including cellulose, starch, and glycogen. It also combines with other compounds to produce common sugars such as sucrose and lactose. The form of D(+)-Glucose displayed above is D-D(+)-Glucose. The “D” designation indicates the configuration of the molecule. The “D” configuration specifies that the hydroxyl group on the number 5 carbon is on the right side of the molecule. The mirror image of D-D(+)-Glucose produces another form of D(+)-Glucose called L-D(+)-Glucose.D(+)-Glucose is the most common form of a large class of molecules called carbohydrates. Carbohydrates are the predominant type of organic compounds found in organisms and include sugar, starches, and fats. Carbohydrates, as the name implies, derive their name from D(+)-Glucose,C6H12O6, which was considered a hydrate of carbon with the general formula of Cn(H2O)n, where n is a positive integer. Although the idea of water bonded to carbon to form a hydrate of carbon was wrong, the term carbohydrate persisted. Carbohydrates consist of carbon, hydrogen, and oxygen atoms, with the carbon atoms generally forming long unbranched chains. Carbohydrates are also known as saccharides derived from the Latin word for sugar, saccharon.화학적 성질
White or almost white, crystalline powder.역사
D(+)-Glucose is the most important and predominant monosaccharide found in nature. It was isolated from raisins by Andreas Sigismund Marggraf (1709–1782) in 1747, and in 1838, Jean-Baptiste-André Dumas (1800–1884) adopted the name glucose from the Greek word glycos meaning sweet. Emil Fischer (1852–1919) determined the structure of glucose in the late 19th century. Glucose also goes by the names dextrose (from its ability to rotate polarized light to the right), grape sugar, and blood sugar. The term blood sugar indicates that glucose is the primary sugar dissolved in blood. Glucose’s abundant hydroxyl groups enable extensive hydrogen bonding, and so glucose is highly soluble in water.용도
D(+)-Glucose anhydrous for biochemistry Reag. Ph Eur. CAS 50-99-7, molar mass 180.16?g/mol.정의
ChEBI: The open chain form of D-glucose.일반 설명
Watery odorless colorless liquid. Denser than water and soluble in water. Hence sinks in and mixes with water.공기와 물의 반응
Water soluble.반응 프로필
A weak reducing agent.건강위험
No toxicitySafety Profile
Mildly toxic by ingest ion. An experimental teratogen. Experi mental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Potentially explosive reaction with potassium nitrate + sodium peroxide when heated in a sealed container. Uxtures with alkali release carbon monoxide when heated. When heated to decomposition it emits acrid smoke and irritating fumes.Purification Methods
Crystallise -D-glucose from hot glacial acetic acid or pyridine. Traces of solvent are removed by drying in a vacuum oven at 75o for >3hours. [Gottfried Adv Carbohydr Chem 5 127 1950, Kjaer & Lindberg Acta Chem Scand 1 3 1713 1959, Whistler & Miller Methods in Carbohydrate Chemistry I 1301962, Academic Press, Beilstein 1 IV 4306.] [For equilibrium forms see Angyal Adv Carbohydr Chem 42 15 1984, Angyal & Pickles Aust J Chem 25 1711 1972.]말토덱스트린 준비 용품 및 원자재
원자재
준비 용품
지베렐린산
리보스
PALATINOSE
Tobacco essence
아버멕틴 B1
High Fructose Syrups
FERROUS GLUCONATE DIHYDRATE
크산탄 검
이소아스코르브산
Glucosyl licorice
Doxycycline hydrochloride
마니톨
페니실린 G 칼륨
alkyl polyglucoside
펙티나아제
5′-구아닐산이나트륨
5'-구아닐산
Cytidine triphosphate
글루콘산칼륨
4-NITROPHENYL-ALPHA-D-GLUCOPYRANOSIDE
AZOXYBENZENE
L-(-) 히스티딘
Vat Yellow 33
O-3-아미노-3-디옥시-알파-D-글루코피라노실-(1→6)-O-[2,6-디아미노-2,3,6-트리디옥시-알파-D-헥소피라노실-(1→4)]-2-디옥시-D-
폴리덱스트로오스
Flour improver
사이클로덱스트린
노르아드레날린
Basic chromic sulfate
COPPER (II) GLUCONATE, MIN. 98
CALCIUM GLUCONATE MONOHYDRATE
Thienamycin
망가니즈글루코네이트
페니실린 감마-일라디산염 세포
글루콘산나트륨
크산토필
에리솔빈산나트륨
POLYOXIN A
클라불란산
γ-사이클로덱스트린
말토덱스트린 공급 업체
글로벌( 1099)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| Jiangsu Guotai Guomian Trading Co. Ltd. | +86-512-58916079 +86-13601562190 |
pharmachem@gtgmt.com | China | 148 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1009 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 |
info@fortunachem.com | China | 5987 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5895 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 2990 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 1143 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 983 | 58 |