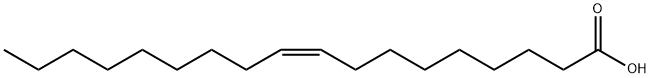
오레인산
|
|
오레인산 속성
- 녹는점
- 13-14 °C(lit.)
- 끓는 점
- 360 °C
- 밀도
- 0.89 g/mL at 25 °C(lit.)
- 증기 밀도
- 1.03 (vs air)
- 증기압
- 52 mm Hg ( 37 °C)
- FEMA
- 2815 | OLEIC ACID
- 굴절률
- n
20/D 1.377
- 인화점
- 133 °F
- 저장 조건
- -20°C
- 용해도
- 에탄올, 에테르, 아세톤, 클로로포름, 디메틸 포름아미드 및 디메틸 설폭시드와 혼합 가능합니다.
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- pKa 5.35(H2O,t =25) (Uncertain)
- Specific Gravity
- 0.892 (20/4℃)
- 색상
- 무색~담황색
- 냄새
- 특이한 라드 같은
- ?? ??
- 지방이 많은
- 수용성
- 무시할 만한
- 감도
- Air Sensitive
- JECFA Number
- 333
- Merck
- 14,6828
- BRN
- 1726542
- Hydrophilic-Lipophilic Balance (HLB)
- 1
- Dielectric constant
- 2.5(20℃)
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 알루미늄과 호환되지 않습니다.
- InChIKey
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N
- LogP
- 7.698 (est)
- CAS 데이터베이스
- 112-80-1(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/24/25-34-40-43-36/37/38-38 | ||
| 안전지침서 | 36/37-37/39-26-36-36/37/39 | ||
| 유엔번호(UN No.) | UN 1198 3/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | LP8925000 | ||
| F 고인화성물질 | 10 | ||
| 자연 발화 온도 | 363°C | ||
| TSCA | Yes | ||
| HS 번호 | 29161500 | ||
| 유해 물질 데이터 | 112-80-1(Hazardous Substances Data) | ||
| 독성 | LD50 i.v. in mice: 230±18 mg/kg (Or, Wretlind) | ||
| 기존화학 물질 | KE-26450 |
오레인산 C화학적 특성, 용도, 생산
용도
비누공업원료,금속광택제.순도시험
(1) 산가 : 이 품목 0.5g을 정밀히 달아 유지류시험법 중 산가에 따라 시험할 때, 196~204이어야 한다.
(2) 응고점 : 이 품목의 응고점은 10℃이하이어야 한다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 요오드가 : 이 품목은 약 0.3g을 정밀히 달은 다음 미리 빙초산․시클로헥산의 혼액(1 : 1) 20mL 및 위이스시액 25mL를 넣어 둔 500mL 공전삼각플라스크에 가해주고 마개를 하고 격렬히 흔들어 준 다음 1시간 어두운 곳에 방치시킨 후 요오드칼륨시액 20mL, 끓여서 식힌 물 100mL를 가하여 과량의 요오드를 0.1N 치오황산나트륨용액으로 적정한다. 이때, 황색이 거의 없어질 때까지 계속 흔들어 주면서 일정하게 0.1N 치오황산나트륨용액을 적가한 다음 다시 전분시액을 가하여 청색이 완전히 없어질 때까지 적정을 계속한다. 종말점 가까이에서는 마개를 하여 격렬히 흔들어 준다. 따로 같은 방법으로 공시험을 행하고 다음 계산식에 따라 요오드가를 구할 때, 그 값은 83~103이어야 한다.
B : 공시험의 0.1N 치오황산나트륨용액의 소비량(mL)
S : 본시험의 0.1N 치오황산나트륨용액의 소비량(mL)
(7) 검화가 : 이 품목 3g을 정밀히 달아 250mL 플라스크에 넣고 0.5N 알콜성수산화칼륨용액 50mL를 가해주고 환류냉각기를 부착한 다음 약 30분 내지 1시간 조용히 검화시킨다. 이 액을 시험용액으로 하여 유지류시험법 중 검화가의 방법에 따라 시험하고, 다시 플라스크의 내용물이 끓을 때까지 가열하고 나타난 홍색이 없어질 때까지 적정하여 검화가를 구할 때, 그 값은 196~206이어야한다.
(8) 불검화물 : 이 품목 5g을 정밀히 달아 250mL 플라스크에 취하고 수산화칼륨 2g 및 에탄올 40mL를 가해주고 환류냉각기를 부착한 다음 조용히 1시간 가열한다. 플라스크의 내용물을 40mL, 80mL, 130mL 눈금이 표시되어 있는 분액여두(길이 30cm, 직경 3.5cm)에 옮기고 플라스크는 충분한 양의 에탄올로 씻어 분액여두에 합하여 40mL로 하고, 다시 더운물과 찬물을 사용하여 플라스크를 씻어 옮겨주고 전량을 80mL로 한다. 마지막으로 석유에테르 수 mL로 플라스크를 씻어 분액여두에 옮겨주고 식힌 다음 석유에테르 50mL를 가해주고 실온이 될 때까지 내용물을 식힌 후 마개를 하고 최소한 1분간 격렬히 진탕한 후 방치하여 두 층으로 분리시킨다. 가능한 한 완전히 분리하고 상층액인 에테르층을 500mL 분액여두에 모은 다음 석유에테르 50mL씩으로 6번 추출한 후 추출액을 처음의 추출액과 합치고 이를 10% 에탄올 25mL로 씻어 주고 물층이 페놀프탈레인시액으로 정색하지 않을 때까지 이 조작을 반복한 후 물층을 완전히 제거한다. 에테르추출액은 미리 무게를 달아둔 비이커에 옮겨주고 에테르 10mL를 사용하여 분액여두를 씻은 다음 비이커에 합해 주고 비이커의 에테르를 수욕상에서 증발건고 한 후 다음 100℃에서 30분간 항량이 될 때까지 건조하고 데시케이타내에서 방냉한 다음 잔류물의 양을 구한다. 이어서 잔류물을 미리 페놀프탈레인시액을 지시약으로 하여 수산화나트륨시액으로 중화시킨 따뜻한 알콜 50mL에 녹이고 0.02N 수산화나트륨용액으로 엷은 홍색이 지속될 때까지 적정하고 그 소비 mL수에 5.659(mg)를 곱하여 올레인산으로서의 양을 구하고 다음 계산식에 따라 불검화물 값을 구할 때, 그 양은 2.0% 이하이어야 한다.
정의
이 품목은 지방에서 얻어지는 불포화지방산으로서 그 주성분은 올레인산(C18H34O2)이다.
강열잔류물
이 품목 10g을 취하여 강열잔류물시험법에 따라 시험할 때, 그 양은 0.01% 이하이어야 한다.
화학적 성질
Oleic acid, C17H33COOH, also known as red oil, elaine oil, and octadecenoic acid, is a yellowish unsaturated fatty acid with an aroma similar to lard. Oleic acid consists chiefly of (Ζ)-9-octadecenoic acid together with varying amounts of saturated and other unsaturated acids. It is insoluble in water, but soluble in most organic solvents. Oleic acid is the main component in cooking and olive oils.It is used for making aluminum oleate, which thickens lubricating oil, and in the preparation of soaps and cosmetics.출처
Reported found in apple, banana, cranberry, guava, grapes, melon, papaya, ginger, hop oil, ginger, beef fat, beer, rum, whiskies, cider, sherry, tea, goat milk, butterfat, celery, cheese, blue cheese, munster cheese, other cheeses, cognac, country cured ham, pork fat, potato, raspberry oil, tomato, peanut oil, coconut meat, avocado, mushroom, fenugreek, tamarind, kelp, cardamom, rice, dill seed, sake, buckwheat, malt, wort, roasted chicory root and cape gooseberry.용도
Oleic acid is a monounsaturated omega-9 fatty acid. Oleic Acid is obtained by the hydrolysis of various animal and vegetable fats and oils. Oleic Acid is used as an emulsifying or solubilizing agent i n aerosol products.생산 방법
Oleic acid is obtained by the hydrolysis of various animal and vegetable fats or oils, such as olive oil, followed by separation of the liquid acids. It consists chiefly of (Ζ)-9-octadecenoic acid. Oleic acid that is to be used systemically should be prepared from edible sources.정의
ChEBI: Oleic acid is an octadec-9-enoic acid in which the double bond at C-9 has Z (cis) stereochemistry. It has a role as an EC 3.1.1.1 (carboxylesterase) inhibitor, an Escherichia coli metabolite, a plant metabolite, a Daphnia galeata metabolite, a solvent, an antioxidant and a mouse metabolite. It is a conjugate acid of an oleate. It derives from a hydride of a cis-octadec-9-ene.일반 설명
Colorless to pale yellow liquid with a mild odor. Floats on water.공기와 물의 반응
Keep cis-9-Octadecenoic acid well closed; protect cis-9-Octadecenoic acid from air and light. . May form peroxides upon exposure to air. This is taken to account for an explosion that occurred, by the mixing of the acid with aluminum, [J. Chem. Educ., 1956, 36, 308]. Water Insoluble.건강위험
Industrial use of compound involves no known hazards. Ingestion causes mild irritation of mouth and stomach. Contact with eyes or skin causes mild irritation.화재위험
cis-9-Octadecenoic acid is combustible.Pharmaceutical Applications
Oleic acid is used as an emulsifying agent in foods and topical pharmaceutical formulations. It has also been used as a penetration enhancer in transdermal formulations,to improve the bioavailability of poorly water-soluble drugs in tablet formulations, and as part of a vehicle in soft gelatin capsules, in topical microemulsion formulations,in oral self-emulsifying drug delivery systems,in oral mucoadhesive patches,and in a metered dose inhaler.Oleic acid was shown to be an important factor in the hypoglycemic effect produced by multiple emulsions containing insulin intended for intestinal delivery of insulin.The phase behavior of sonicated dispersions of oleic acid has been described,and mechanisms for the topical penetrationenhancing actions of oleic acid have been presented.
Oleic acid has been reported to act as an ileal ‘brake’ that slows down the transit of luminal contents through the distal portion of the small bowel.
Oleic acid labeled with 131I and 3H is used in medical imaging.
Safety Profile
Poison by intravenous route. Mildly toxic by ingestion. Mutation data reported. A human skin and eye irritant. Questionable carcinogen with experimental tumorigenic data. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical. The peroxidzed acid explodes on contact with aluminum. Potentially dangerous reaction with perchloric acid + heat. When heated to decomposition it emits acrid smoke and irritating fumes.Safety
Oleic acid is used in oral and topical pharmaceutical formulations.In vitro tests have shown that oleic acid causes rupture of red blood cells (hemolysis), and intravenous injection or ingestion of a large quantity of oleic acid can therefore be harmful. The effects of oleic acid on alveolar and buccal epithelial cells in vitro have also been studied; the in vitro and in vivo effects of oleic acid on rat skin have been reported. Oleic acid is a moderate skin irritant; it should not be used in eye preparations.
An acceptable daily intake for the calcium, sodium, and potassium salts of oleic acid was not specified by the WHO since the total daily intake of these materials in foods was such that they did not pose a hazard to health.
LD50 (mouse, IV): 0.23 g/kg
LD50 (rat, IV): 2.4 mg/kg
LD50 (rat, oral): 74 g/kg
Carcinogenicity
Some recent studies suggested that oleic acid may decrease the incidence of mammary gland tumors of some rodent species. In a reviewof several fatty acids, Ip concludes that there is little evidence for the protective effect of oleic acid on the development of cancer.저장
On exposure to air, oleic acid gradually absorbs oxygen, darkens in color, and develops a more pronounced odor. At atmospheric pressure, it decomposes when heated at 80–100°C.Oleic acid should be stored in a well-filled, well-closed container, protected from light, in a cool, dry place.
Purification Methods
Purify the acid by fractional crystallisation from its melt, followed by molecular distillation at 10 -3mm, or by conversion to its methyl ester, the free acid can be crystallised from acetone at -40o to -45o (12mL/g). For purification by the use of lead and lithium salts, see Keffler and McLean [J Soc Chem Ind (London) 54 176T 1935]. Purification based on direct crystallisation from acetone is described by Brown and Shinowara [J Am Chem Soc 59 6 1937, pK White J Am Chem Soc 72 1857 1950]. [Beilstein 2 H 463, 2 I 198, 2 II 429, 2 III 1387, 2 IV 1641.]비 호환성
Incompatible with aluminum, calcium, heavy metals, iodine solutions, perchloric acid, and oxidizing agents. Oleic acid reacts with alkalis to form soaps.Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (inhalation and nasal aerosols, tablets, topical and transdermal preparations). Included in nonparenteral medicines (metered dose inhalers; oral capsules; oral prolonged release granules; topical creams and gels) licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.오레인산 준비 용품 및 원자재
원자재
준비 용품
오레인산 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co., Ltd. | +8619930503259 |
cherry@crovellbio.com | China | 18420 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 |
Sibel@weibangbio.com | China | 5848 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 |
admin@86-ss.com | China | 1000 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 |
L18532138899@163.com | China | 931 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 |
sales06@hbduling.cn | China | 8055 | 58 |
| Alfa Chemistry | |
Info@alfa-chemistry.com | United States | 24072 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5873 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 80 | 58 |