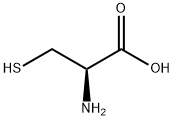
L-시스테인
|
|
L-시스테인 속성
- 녹는점
- 240 °C (dec.) (lit.)
- 끓는 점
- 293.9±35.0 °C(Predicted)
- 알파
- 8.75 º (c=12, 2N HCl)
- 밀도
- 1.197 (estimate)
- 굴절률
- 8.8 ° (C=8, 1mol/L HCl)
- FEMA
- 3263 | L-CYSTEINE
- 저장 조건
- Store below +30°C.
- 용해도
- H2O: 25 mg/mL
- 산도 계수 (pKa)
- 1.92(at 25℃)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- 수소이온지수(pH)
- 4.5-5.5 (100g/l, H2O, 20℃)
- 냄새
- 유황의
- ?? ??
- 유황의
- optical activity
- Optical rotation: +8° to +9° (c = 5, 1 N HCl, 20°C).
- 수용성
- 280g/L(25℃)
- 감도
- Air Sensitive
- 최대 파장(λmax)
- λ: 260 nm Amax: 1.5
λ: 280 nm Amax: 0.2
- JECFA Number
- 1419
- Merck
- 14,2781
- BRN
- 1721408
- 안정성
- 안정성 안정적이지만 공기에 민감할 수 있습니다. 산화제, 염기와 호환되지 않습니다.
- LogP
- -2.49
- CAS 데이터베이스
- 52-90-4(CAS DataBase Reference)
- NIST
- L-Cysteine(52-90-4)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36/37/38-20/21/22 | ||
| 안전지침서 | 36-37/39-26-24/25 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | HA1600000 | ||
| F 고인화성물질 | 10-23 | ||
| 자연 발화 온도 | 420 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29309012 | ||
| 유해 물질 데이터 | 52-90-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 1890 mg/kg | ||
| 기존화학 물질 | KE-01607 |
L-시스테인 C화학적 특성, 용도, 생산
물성
무색∼흰색의 결정 또는 흰백색의 결정성 가루로 특이한 냄새와 맛이 있다. 물에 녹으며 메탄올에 잘 용해되나 에테르에 용해되지 않는다.물성
L-시스틴과 달리 물, 에탄올에 녹는다. 니트로프루시드 반응, 설리반 반응 등 양성. 수은, 구리 등과 불용성의 염을 만든다. 중성 또는 약한 알칼리성으로 철 또는 구리 이온의 존재에서 공기에 의해 L-시스틴으로 산화되고 브롬에서는 더욱 산화된 L-시스테산을 생성한다. 알칼리성 용액 중에서는 매우 불안정하여 황화수소, 암모니아를 발생하여 피루브산을 생성한다. 염산의 존재에서 알데히드, 케톤류와 축합하여 상당하는 티오아세탈, 케톤티오아세탈을 생성하고 중성에서 가열한 경우에는 티아졸리딘 유도체가 된다.용도
산화방지제, 착향제, 퍼머넌트웨이브용제/헤어스트레이트너용제, 헤어개요
Cysteine (abbreviated as Cys or C) is an α-amino acid with the chemical formula HO2CCH(NH2)CH2SH. It is a semi - essential amino acid, which means that it can be biosynthesized in humans. The thiol side chain in cysteine often participates in enzymatic reactions, serving as a nucleophile. The thiol is susceptible to oxidization to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920.화학적 성질
A sulfur-containing amino acid, metabolically related to methionine. Methionine is the source of sulfur atom in the synthesis of cysteine in the body. Chemically, L-cysteine is L-2-amino-mercaptopropionic acid. Cysteine has a sulfureous aroma. It is a nutrient and is used in dietary supplements.물리적 성질
Solubility 28 (25 ℃) g/100 mL solution, 16 (20 ℃) g/100 g H2O, pI 5.02, dissociation constants: pK1 1.71, pK2 8.27 (–SH), pK3 10.78.출처
Dietary sourcesAlthough classified as a non-essential amino acid, in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available.
Cysteine is catabolized in the gastrointestinal tract and blood plasma. In contrast, cystine travels safely through the GI tract and blood plasma and is promptly reduced to the two cysteine molecules upon cell entry.
Industrial sources
The majority of L - cysteine is obtained industrially by hydrolysis of poultry feathers or human hair. Synthetically produced L-cysteine, compliant with Jewish Kosher and Muslim Halal rules, is also available, albeit at a higher price. The synthetic route involves fermentation utilizing a mutant of E. coli.
Biosynthesis
In animals, biosynthesis begins with the amino acid serine. The sulfur is derived from methionine, which is converted to homocysteine through the intermediate S- adenosylmethionine. Cystathionine betasynthase then combines homocysteine and serine to form the asymmetrical thioether cystathionine. The enzyme cystathionine gamma-lyase converts the cystathionine into cysteine and alphaketobutyrate.
용도
L-Cysteine is a non-essential amino acid that can be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available. L-Cysteine is commonly used as a precursor in the food and pharmaceutical industries. L-Cysteine is used as a processing aid for baking, as an additive in cigarettes, as well as in the preparation of meat flavours. It is used in foods to prevent oxygen from destroying vitamin c and is used in doughs to reduce mixing time.주요 응용
Cysteine, mainly the L-enantiomer, is a precursor in the food, pharmaceutical, and personal care industries. One of the largest applications is the production of flavors. For example, the reaction of cysteine with sugars in a Maillard reaction yields meat flavors. Lcysteine is also used as a processing aid for baking.In the field of personal care, cysteine is used for permanent wave applications predominantly in Asia. Again the cysteine is used for breaking up the disulfide bonds in the hair's keratin.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics. Maleimides will selectively attach to cysteine using a covalent Michael addition. Site- directed spin labeling for EPR or paramagnetic relaxation enhanced NMR also uses cysteine extensively.
cysteine is an essential amino acid obtained by fermentation. Cysteine is a component of the skin’s natural moisturizing factor and can help normalize oil gland secretion because of its sulfur content. It is also said to promote wound healing. In addition, studies indicate that cysteine helps increase levels of glutathione (an anti-oxidant) in the body. It is considered beneficial in treating oily skin.
제조 방법
L-Cysteine used to be produced almost exclusively by hydrolysis of hair or other keratins. The amino acid isolated was l-cystine, which was reduced electrolytically to l-cysteine. L-Cysteine has also been prepared from beta-chloro-d,l-alanine and sodium sulfide with cysteine desulfhydrase, an enzyme obtained from, e.g., Citrobacterium freundii. Today, however, the main processes for cysteine production are biological. A direct fermentation process has been developed for the manufacture of l-cystine, using a modified Escherichia coli bacterium. The technology has been extended to prepare other modified l-cysteine analogues. An enzymatic process for l-cysteine has been successfully developed using microorganisms capable to hydrolyze 2-amino-delta2-thiazoline 4-carboxylic acid (ATC) which is readily available from methyl alpha-chloroacrylate and thiourea. A mutant of Pseudomonas thiazolinophilum converts d,l-ATC to l-cysteine in 95% molar yield at product concentrations higher than 30 g/L.정의
ChEBI: L-cysteine is an optically active form of cysteine having L-configuration. It has a role as a flour treatment agent, a human metabolite and an EC 4.3.1.3 (histidine ammonia-lyase) inhibitor. It is a serine family amino acid, a proteinogenic amino acid, a cysteine and a L-alpha-amino acid. It is a conjugate base of a L-cysteinium. It is a conjugate acid of a L-cysteinate(1-). It is an enantiomer of a D-cysteine. It is a tautomer of a L-cysteine zwitterion.일반 설명
L-cysteine is a sulfur-containing non-essential amino acid. Its ability to reduce colitis symptoms is being assessed for potential use in treating inflammatory bowel disease (IBD).부작용
Cysteine has been proposed as a preventative or antidote for some of the negative effects of alcohol, including liver damage and hangover. It counteracts the poisonous effects of acetaldehyde, which is the major by - product of alcohol metabolism and is responsible for most of the negative aftereffects and long - term damage associated with alcohol use (but not the immediate effects of drunkenness). Cysteine supports the next step in metabolism, which turns acetaldehyde into the relatively harmless acetic acid. In a rat study, test animals received an LD50 dose of acetaldehyde. Those that received cysteine had an 80 % survival rate; when both cysteine and thiamine were administered, all animals survived . There is not yet direct evidence for or against its effectiveness in humans who consume alcohol at normal levels.N-Acetylcysteine
N - Acetyl - L - cysteine (NAC) is a derivative of cysteine wherein an acetyl group is attached to the nitrogen atom. This compound is sold as a dietary supplement and used as an antidote in cases of acetaminophen overdose, and obsessive compulsive disorders such as trichotillomania.
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Experimental reproductive effects. Human mutation data reported. When heated to decomposition fumes of SO and NO.Purification Methods
Purify it by recrystallisation from H2O (free from metal ions) and dry it in a vacuum. It is soluble in H2O, EtOH, Me2CO, EtOAc, AcOH, *C6H6 and CS2. Acidic solutions can be stored under N2 for a few days without deterioration. [For synthesis and spectra see Greenstein & Winitz Chemistry of the Amino Acids (J. Wiley) Vol 3 p1879 1961, Beilstein 4 III 1618, 4 IV 3144.]L-시스테인 준비 용품 및 원자재
원자재
준비 용품
L-시스테인 공급 업체
글로벌( 1054)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ENBRIDGE PHARMTECH CO., LTD. | +8613812269233 |
tinayang@enbridgepharm.com | China | 302 | 58 |
| HAINAN POLY PHARMCEUTICAL CO. LTD | +86-0571-89385087 +8613616530509 |
ff@hnpoly.com | China | 118 | 58 |
| DONBOO AMINO ACID COMPANY | +8613063595538 |
donboo@donboo.com | China | 9365 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Wuhan Fortuna Chemical Co.,Ltd | +8618007136271 |
hk@fortunachem.com | China | 5984 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5886 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 988 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 2990 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |