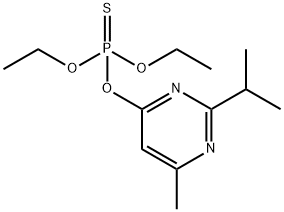
다이아지논
|
|
다이아지논 속성
- 녹는점
- >120°C (dec.)
- 끓는 점
- 306°C
- 밀도
- 1.117
- 증기압
- 1.2 x 10-2 Pa (25 °C)
- 굴절률
- nD20 1.4978-1.4981
- 인화점
- 104.4 °C
- 저장 조건
- APPROX 4°C
- 용해도
- Chloroform: Slightly Soluble
- 산도 계수 (pKa)
- 1.21±0.30(Predicted)
- 물리적 상태
- Liquid
- 색상
- Light brown to brown
- 수용성
- 약간 용해됨. 0.004g/100mL
- Merck
- 13,3019
- BRN
- 273790
- 노출 한도
- OSHA PEL: TWA 0.1 mg/m3; ACGIH TLV: TWA 0.1 mg/m3.
- 안정성
- 수분에 민감한
- CAS 데이터베이스
- 333-41-5(CAS DataBase Reference)
- IARC
- 2A (Vol. 112) 2017
- NIST
- Diazinone(333-41-5)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn;N,N,Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-50/53-36-20/21/22-11-51/53 | ||
| 안전지침서 | 24/25-60-61-36-26-16-36/37 | ||
| 유엔번호(UN No.) | UN 2783/2810 | ||
| OEB | D | ||
| OEL | TWA: 0.1 mg/m3 [skin] | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TF3325000 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 29335990 | ||
| 유해 물질 데이터 | 333-41-5(Hazardous Substances Data) | ||
| 독성 | LD50 in male, female rats (mg/kg): 250, 285 orally (Gaines) | ||
| 기존화학 물질 | KE-28690 | ||
| 유해화학물질 필터링 | 97-1-14 | ||
| 중점관리물질 필터링 | 별표1-82 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 다이아지논 및 이를 1% 이상 함유한 혼합물 |
다이아지논 C화학적 특성, 용도, 생산
개요
Diazinon is a non-systemic organophosphate insecticide formerly used to control cockroaches, silverfish, ants, and fleas in residential, non-food buildings. Diazinon functions as the inhibitor of acetylcholinesterase (AChE), which breaks down the neurotransmitter acetylcholine (ACh) into [choline] and an acetate group. The inhibition of the AChE causes an abnormal accumulation of ACh in the synaptic cleft. However, recent studies have shown that diazinon and other kinds of organophosphate can cause neural toxicity through causing oxidative stress in the neural cells.화학적 성질
Diazinon is available as a colorless or dark brown liquid. It is sparingly soluble in water but very soluble in petroleum ether, alcohol, and benzene. Diazinon is used for the control of a variety of agricultural and household pests. These include pests in soil, on ornamental plants, fruit, vegetable, crops pests, and household pests like fl ies, fl eas, and cockroaches. Diazinon undergoes decomposition on heating above 120°C and produces toxic fumes, such as nitrogen oxides, phosphorous oxides, and sulfur oxides. It reacts with strong acids and alkalis with the possible formation of highly toxic tetra ethyl thiopyrophosphates. Diazinon is classifi ed as an RUP. Depending on the type of formulation, diazinon is classifi ed as toxicity class II, meaning moderately toxic, or toxicity class III, meaning slightly toxic.용도
Diazinon is used to control a wide range of sucking and chewing insects and mites in a very wide range of crops and is also used as a veterinary ectoparasiticide.정의
ChEBI: A member of the class of pyrimidines that is pyrimidine carrying an isopropyl group at position 2, a methyl group at position 6 and a (diethoxyphosphorothioyl)oxy group at position 4.일반 설명
Diazinon is available in the form of a colourless or dark brown liquid. It is sparingly soluble in water but very soluble in petroleum ether, alcohol, and benzene. Diazinon is used for the control of a variety of agriculture and household pests. These include pests in soil, on ornamental plants, fruit, vegetable, and crops and household pests like flies, fleas, and cockroaches. Diazinon undergoes decomposition on heating above 120°C and produces toxic fumes such as nitrogen oxides, phosphorus oxides, and sulphur oxides. It reacts with strong acids and alkalis with possible formation of highly toxic tetra ethyl thiopyrophosphates. Diazinon is classified as a RUP. Depending on the type of formulation, diazinon is classified as toxicity class II, meaning moderately toxic, or toxicity class III, meaning slightly toxic.공기와 물의 반응
The neat compound is susceptible to oxidation and should be protected from prolonged exposure to air . Insoluble in water.건강위험
Humans are exposed to diazinon during manufacture and professional applications. Diazinon causes poisoning with symptoms such as headache, dizziness, nausea, weakness, feelings of anxiety, vomiting, pupillary constriction, convulsions, respiratory distress or labored breathing, unconsciousness, muscle cramp, excessive salivation, respiratory failure, and coma.화재위험
Not flammable. POISONOUS GASES ARE PRODUCED WHEN HEATED. Oxides of sulfur and of phosphorus are generated in fires.상품명
AG-500®; AI3-19507®; ALFA-TOX®[C]; ANTIGAL®; ANTLAK®; BASUDIN®; BAZUDEN®; CASWELL No. 342®; DACUTOX®; DASSITOX®; DAZZEL®; DIAGRAN®; DIANON®; DIATERR-FOS®; DIAZAJET®; DIAZATOL®; DIAZIDE®; DIAZINON AG 500 WBC®; DIAZINONE®; DIAZITOL®; DIAZOL®; DICID®; DIMPYLATE®; DIPOFENE®; DIZIKTOL®; DIZINON®[C]; DRAWIZON®; DYMET®; DYZOL®); D.Z.N.®; EXODIN®; FEZUDIN®; FLYTROL®; G 301®; G-24480®; GALESAN®; GARDENTOX®; GEIGY 24480®; KAYAZINON®; KAYAZOL®; NEOCIDOL® (OIL); NEOCIDOL®; NIPSAN®; NUCIDOL®; OLEODIAZINON®; ROOT GUARD; SAROLEX®[C]; SPECTRACIDE®; SROLEX®; SUZON®Safety Profile
Poison by ingestion, skin contact, subcutaneous, intravenous, and intraperitoneal routes. Mildly toxic by inhalation. Human systemic effects by ingestion: changes in motor activity, muscle weakness, and sweating. Experimental teratogenic and reproductive effects. A skin and severe eye irritant. Human mutation data reported. When heated to decomposition it emits very toxic fumes of NOx, POx, and SOx.잠재적 노출
roducers, formulators and applicators of this nonsystemic pesticide and acaricide. Diazinon is used in the United States on a wide variety of agricultural crops, ornamentals, domestic animals; lawns and gardens; and household pests.Carcinogenicity
Among 23,106 male applicators participating in the Agricultural Health Study who reported using diazinon, there was an increased risk with exposure to diazinon for lung cancer, leukemia, and all cancer sites combined, although the small number of cases observed makes these estimates unreliable .신진 대사 경로
The main route of diazinon metabolism in soil, plants and animals is through cleavage of the P-O-pyrimidine group to yield 2-isopropyl-4- methyl-6-hydroxypyrimidine. As with most other phosphorothioates, loss of the pyrimidinyl function in mammalian metabolism probably occurs either through oxidative desulfuration of the thiono group, catalysed by microsomal mixed function oxidases, to give diazoxon followed by hydrolysis catalysed by an A-esterase, or via an oxidative mechanism catalysed by a mixed function oxidase acting directly on diazinon. In the first case the second product is diethyl phosphate and in the second, diethyl phosphorothioate (Yang et al., 1971). Further metabolism then leads to hydroxylation of the methyl and isopropyl groups on the pyrimidine ring. This oxidative metabolism may the act on the pyrimidinol, diazoxon or diazinon itself, the last of which seems to be important in mammalian and avian liver and gives rise to metabolites which still have anticholinesterase or latent anticholinesterase activity.신진 대사
The main biodegradation pathway in mammals, plants, and soils is pyrimidinyl ester bond cleavage; the principal metabolites are diethyl phosphorothioate and diethyl phosphate. Degradation in the environment involves oxidation to diazoxon and hydrolysis.운송 방법
UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.비 호환성
Reaction with nitrosating agents (e.g., nitrites, nitrous gases, nitrous acid) capable of releasing carci- nogenic nitrosamines. Hydrolyzes slowly in water and dilute acid. Reacts with strong acids and alkalis with possible forma- tion of highly toxic tetraethyl thiopyrophosphates. Incompatible with copper-containing compounds. Contact with oxidizers may cause the release of phosphorous oxides. Contact with strong reducing agents, such as hydrides; may cause the formation of flammable and toxic phosphine gas.폐기물 처리
Diazinon is hydrolyzed in acid media about 12 times as rapidly as parathion, and at about the same rate as parathion in alkaline media. In excess water this compound yields diethylthiophosphoric acid and 2-isopropyl-4-methyl-6-hydroxypyrimidine. With insufficient water, highly toxic tetraethyl monothiopyropho- sphate is formed. Therefore, incineration would be a prefer- able ultimate disposal method with caustic scrubbing of the incinerator effluent . In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by follow- ing package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.주의 사항
Workers should avoid eye contact with diazinon, wear chemical safety glasses or goggles, protective clothing or equipment, wear waterproof boots, long-sleeved shirts, long pants, and a hat. Workers should avoid contamination of food and feed, wash thoroughly after handling and before eating or smoking. In fact, occupational workers should avoid eating, drinking, or smoking in areas of work with the chemical.참고 문헌
Uner, N, et al. "Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus." Environmental Toxicology & Pharmacology 21.3(2006):241.Shishido, Takashi, K. Usui, and J. I. Fukami. "Oxidative metabolism of diazinon by microsomes from rat liver and cockroach fat body." Pesticide Biochemistry & Physiology 2.1(1972):27-38.
Giordano, G, et al. "Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency." Toxicology & Applied Pharmacology 219.2-3(2007):181.
다이아지논 준비 용품 및 원자재
원자재
아세트초산에틸
Ancymidol
비스(2-디메틸아미노에틸)에테르
6-Methylpyrimidine
메틸 아세토아세테이트
BUTYRAMIDINE
2-이소프로필-6-메틸-4-피리미디놀
디에틸 티오포스포릴 염화물
퀴날포스
부소부틸로니트릴
피리딘 -2,4- 다이 카복실산
중탄산나트륨(중조)
준비 용품
다이아지논 공급 업체
글로벌( 278)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Klong Industrial Co., Ltd | 0086-519-68231162 |
info@klongchem.com | CHINA | 131 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49392 | 58 |