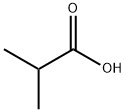
이소낙산
|
|
이소낙산 속성
- 녹는점
- -47 °C (lit.)
- 끓는 점
- 153-154 °C (lit.)
- 밀도
- 0.95 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.04 (vs air)
- 증기압
- 1.5 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.393(lit.)
- 인화점
- 132 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 618g/l
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 4.84(at 20℃)
- 색상
- 무색의
- 수소이온지수(pH)
- 3.96(1 mM solution);3.44(10 mM solution);2.93(100 mM solution);
- 냄새
- 프로필렌 글리콜 중 1.00%. 산성 신 치즈 낙농 버터 같은 썩은 냄새
- ?? ??
- 산성의
- Odor Threshold
- 0.0015ppm
- 폭발한계
- 1.6-7.3%(V)
- 수용성
- 210g/L(20℃)
- Merck
- 14,5155
- JECFA Number
- 253
- BRN
- 635770
- Dielectric constant
- 2.6(Ambient)
- InChIKey
- KQNPFQTWMSNSAP-UHFFFAOYSA-N
- LogP
- 1.1 at 25℃
- CAS 데이터베이스
- 79-31-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 21/22 | ||
| 안전지침서 | 23-36/37/39-24/25 | ||
| 유엔번호(UN No.) | UN 2529 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | NQ4375000 | ||
| F 고인화성물질 | 13 | ||
| 자연 발화 온도 | 824 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29156000 | ||
| 유해 물질 데이터 | 79-31-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 266 mg/kg LD50 dermal Rabbit 475 mg/kg | ||
| 기존화학 물질 | KE-24875 |
이소낙산 C화학적 특성, 용도, 생산
화학적 성질
Isobutyric acid is a clear colorless oily liquid with an odor and flavor similar to n-butyric acid. Miscible with water, soluble in ethanol and ether. Prepared via oxidation of isobutyl alcohol.물리적 성질
Isobutyric Acid is a flavoring agent that is a colorless liquid with a strong, penetrating odor, resembling butter. it is miscible in alcohol, propylene glycol, glycerin, mineral oil, and most fixed oils and soluble in water. it is obtained by chemical synthesis. it is also termed isopropylformic acid.출처
Isobutyric acid occurs naturally in Ceratonia siliqua L. The gum obtained from the kernels of this species is used as a thickener in the food industry. Reported found in several essential oils: Arnica montana, Roman chamomile, Laurus nobilis, imperatoria, and in carob fruits (Siliqua dulcis); also identified in the essence of Seseli tortuosum, Artemisia transiliensis.제조 방법
Isobutyric acid is prepared in a similar way to butyric acid, mainly by direct oxidation of isobutanol and isobutyraldehyde, which is obtained by a direct oxidation reaction with air or oxygen.정의
ChEBI: Isobutyric acid is a branched fatty acid comprising propanoic acid carrying a methyl branch at C-2. It has a role as a volatile oil component, a plant metabolite and a Daphnia magna metabolite. It is a branched-chain saturated fatty acid, a methyl-branched fatty acid and a fatty acid 4:0. It is a conjugate acid of an isobutyrate.주요 응용
Isobutyric acid is mainly used in the synthesis of isobutyric acid esters, such as methyl isobutyrate, propyl ester, isoamyl ester and benzyl ester. It can also be used manufacture of esters for solvents, flavors and perfume bases, disinfecting agent, varnish, plasticizers, deliming hides, tanning agent and used in pharmaceutical. Isobutyric acid has some important derivatives that, in the industry, is actually used for the production of isobutyronitrile intermediates, and then converted to isobutylamidine hydrochloride that is the raw materials of pesticide diazinon.일반 설명
Isobutyric acid appears as a colorless liquid with a light odor of rancid butter. Flash point 132°F. Density 7.9 lb / gal. Corrosive to metals and tissue.공기와 물의 반응
Flammable. Water soluble반응 프로필
Isobutyric acid corrodes aluminum and other metals. Flammable hydrogen gas may accumulate in enclosed spaces in which this reaction has taken place [USCG, 1999].위험도
Toxic by ingestion, strong irritant to tissue.건강위험
Inhalation causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes irritation.화재위험
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.Synthesis
The preparation of isobutyric acid is similar with butyric acid, which is performed by the direct oxidation of isobutyl alcohol and isobutyraldehyde. Isobutyric acid can be directly generated from the oxidation of isobutyraldehyde in air or oxygen. Other manufacturing methods have isobutyronitrile hydrolysis and methacrylic acid hydrogenation. The oxidation of 2-methyl-1-nitropropane to prepare isobutyric acid can also obtain a higher yield. The purification of Isobutyric acid can be realized by azeotropic distillation with water, and anhydrous isobutyric acid can be obtained by the extractive distillation from carbon tetrachloride. Propylene and formic acid ester can react at 50 °C with the catalysis of hydrofluoric acid to generate methyl isobutyrate and propyl isobutyrate.Purification Methods
Distil the acid from KMnO4, then redistil it from P2O5. [Beilstein 2 H 288, 2 I 126, 2 II 257, 2 III 637, 2 IV 843.]이소낙산 준비 용품 및 원자재
원자재
몰식자산프로필
하이드라진 수화물
OCTYL ISOBUTYRATE
이소부탄올
부탄 산
1-니트로프로판
이소부틸산메틸에스테르
Propyl isobutyrate
BUTYRAMIDINE
Isopentyl isobutyrate
사염화탄소
Benzyl isobutyrate
AROMA
(-)-MENTHYL CHLOROFORMATE
다이아지논
이소부틸알데히드
불산
메타크릴 산
부소부틸로니트릴
준비 용품
ethyl 3,3-dimethylpent-4-en-1-oate
부틸 이소부틸레이트
Hexyl isobutyrate
Phenethyl isobutyrate
아이소-뷰틸 폼산
OCTYL ISOBUTYRATE
HEPTYL ISOBUTYRATE
2-(4-Ethoxyphenyl)-2-methylpropanol
이소부틸산메틸에스테르
ethyl 4,6,6-trichloro-3,3-dimethyl-hex-5-enoate
CINNAMYL ISOBUTYRATE
t-부틸 퍼옥시피발레이트
에틸 2-브로모아이소뷰티레이트
캅토프릴
Benzyl isobutyrate
Ethyl 2-(2-aminothiazole-4-yl)-2-(1-tert-butoxycarbonyl-1-methylethoxyimino)acetate
P-TOLYL ISOBUTYRATE
무수이소부틸산
퍼메트린
이소부티르아미드
CITRONELLYL ISOBUTYRATE
4-(3-ISOPROPYL-1,2,4-OXADIAZOL-5-YL)PIPERIDINE
2-브롬-2-메틸프로피오닐브로마이드
에토펜프록스
이소부티릴 클로라이드
에틸이소부틸레이트
겜핍로질
페녹시에틸 아이소부티레이트
이소낙산 공급 업체
글로벌( 473)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |