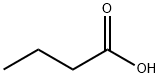
부탄 산
|
|
부탄 산 속성
- 녹는점
- ?6-?3 °C (lit.)
- 끓는 점
- 162 °C (lit.)
- 밀도
- 0.964 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.04 (vs air)
- 증기압
- 0.43 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.398(lit.)
- FEMA
- 2221 | BUTYRIC ACID
- 인화점
- 170 °F
- 저장 조건
- Store below +30°C.
- 용해도
- Chloroform (Soluble), Isopropanol (Sparingly), Methanol (Slightly);Miscible with water, Propylene glycol, Glycerin, alcohol and oils.
- 산도 계수 (pKa)
- 4.83(at 25℃)
- 물리적 상태
- 액체
- Specific Gravity
- 0.960 (20/4℃)
- 색상
- 무색의
- 냄새
- 디프로필렌 글리콜 중 1.00%. 날카로운 아세트 치즈 버터 과일
- 수소이온지수(pH)
- 3.94(1 mM solution);3.42(10 mM solution);2.92(100 mM solution);
- ?? ??
- 치즈맛이 나는
- Odor Threshold
- 0.00019ppm
- 폭발한계
- 2-12.3%(V)
- 수용성
- 혼용 가능
- Merck
- 14,1593
- JECFA Number
- 87
- BRN
- 906770
- Dielectric constant
- 3.0(Ambient)
- 안정성
- 가연성. 강한 산화제, 알루미늄 및 대부분의 기타 일반 금속, 알칼리, 환원제와 호환되지 않습니다.
- InChIKey
- FERIUCNNQQJTOY-UHFFFAOYSA-N
- LogP
- 1.1 at 25℃
- CAS 데이터베이스
- 107-92-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34 | ||
| 안전지침서 | 26-36-45 | ||
| 유엔번호(UN No.) | UN 2820 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | ES5425000 | ||
| F 고인화성물질 | 13 | ||
| 자연 발화 온도 | 824 °F | ||
| 위험 참고 사항 | Irritant | ||
| TSCA | Yes | ||
| HS 번호 | 2915 60 19 | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 107-92-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 8.79 g/kg (Smyth) | ||
| 기존화학 물질 | KE-03838 |
부탄 산 C화학적 특성, 용도, 생산
순도시험
(1) 비중 : 이 품목의 비중은 0.952~0.956이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 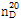 은 1.397~1.399이어야 한다.
은 1.397~1.399이어야 한다.
(3) 황산염 : 이 품목 10g을 취해 황산염시험법에 따라 시험할 때, 그 양은 0.01N 황산 0.4mL에 대응하는 양 이하이어야 한다.
확인시험
(1) 이 품목 1mL에 물 2mL를 넣으면 투명하게 녹는데 그 액은 강산성이다.
(2) 이 품목 1mL에 에탄올 1mL 및 황산 3방울을 넣어 온탕 중에서 가온하면 낙산에틸향기가 발생된다.
정량법
이 품목 약 1.5g을 정밀히 달아 물 75mL를 넣어 0.5N 수산화나트륨용액으로 적정한다(지시약 : 페놀프탈레인시액 2방울).
0.5N 수산화나트륨용액 1mL = 44.06mg C4H8O2
개요
Butyric acid is a carboxylic acid also classified as a fatty acid. It exists in two isomeric forms as shown previously, but this entry focuses on n-butyric acid or butanoic acid. It is a colorless, viscous, rancid-smelling liquid that is present as esters in animal fats and plant oils. Butyric acid exists as a glyceride in butter, with a concentration of about 4%; dairy and egg products are a primary source of butyric acid. When butter or other food products go rancid, free butyric acid is liberated by hydrolysis, producing the rancid smell. It also occurs in animal fat and plant oils.화학적 성질
Butyric acid, C3H7COOH, a colorless liquid with an obnoxious odor, occurring in spoiled butter.It miscible with water, alcohol, and ether.It is used in the synthesis of butyrate ester perfume and flavor ingredients and in disinfectants and pharmaceuticals,출처
Normally occurs in butter as a glyceride. It has been reported found in the essential oils of citronella Ceylon, Eucalyptus globules, Araucaria cunninghamii, Lippia scaberrima, Monarda fistulosa, cajeput, Heracleum giganteum, lavender, Hedeoma pulegioides, valerian, nutmeg, hops, Pastinaca sativa, Spanish anise and others. It has been identified in strawberry aroma, apricot, American cranberry, sour cherry, black currants, butter, milk, strawberry jam, cheeses (blue, cheddar, feta, Swiss, Camembert and romano), raspberry, papaya, coffee mutton, beer, rum, bourbon whiskey and cider.역사
Butyric acid gets its name from the Latin butyrum, or butter. It was discovered by Adolf Lieben (1836–1914) and Antonio Rossi in 1869. Butyric acid is one of the simplest fatty acids.용도
Butyric Acid is a fatty acid that is commonly obtained from butter fat. it has an objectionable odor which limits its uses as a food acid- ulant or antimycotic. it is an important chemical reactant in the manufacture of synthetic flavoring, shortening, and other edible food additives. in butter fat, the liberation of butyric acid which occurs during hydrolytic rancidity makes the butter fat unusable. it is used in soy milk-type drinks and candies.생산 방법
Butyric acid is produced by oxidation of butyraldehyde (CH3(CH2)2CHO) or butanol (C4H9OH). It can also be formed biologically by the oxidation of sugar and starches using bacteria.제조 방법
Obtained by fermentation of starches and molasses with selective enzymes (Granulo saccharobutyricum); it is subsequently isolated as the calcium salt.정의
ChEBI: A straight-chain saturated fatty acid that is butane in which one of the terminal methyl groups has been oxidised to a carboxy group.일반 설명
A colorless liquid with a penetrating and unpleasant odor. Flash point 170°F. Corrosive to metals and tissue. Density 8.0 lb /gal.공기와 물의 반응
Water soluble.반응 프로필
(3R,4S)-1-Benzoyl-3-(1-methoxy-1-methylethoxy)-4-phenyl-2-azetidinone can react with oxidizing agents. Incandescent reactions occur with chromium trioxide above 212°F. Also incompatible with bases and reducing agents. May attack aluminum and other light metals .위험도
Strong irritant to skin and tissue.건강위험
Inhalation causes irritation of mucous membrane and respiratory tract; may cause nausea and vomiting. Ingestion causes irritation of mouth and stomach. Contact with eyes may cause serious injury. Contact with skin may cause burns; chemical is readily absorbed through the skin and may cause damage by this route.화재위험
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Biotechnological Applications
Butyrate is produced as end - product of a fermentation process solely performed by obligate anaerobic bacteria. Fermented Kombucha "tea" includes butyric acid as a result of the fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861.The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is then oxidized into acetyl coenzyme A using a unique mechanism that involves an enzyme system called pyruvate - ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of elemental hydrogen (H2) are formed as waste products from the cell.
Safety Profile
Moderately toxic by ingestion, skin contact, subcutaneous, intraperitoneal, and intravenous routes. Human mutation data reported. Severe skin and eye irritant. A corrosive material. Combustible liquid. Could react with oxidizing materials. Incandescent reaction with chromium trioxide above 100'. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.Safety
The United States Environmental Protection Agency rates and regulates butyric acid as a toxic substance.Personal protective equipment such as rubber or PVC gloves, protective eye goggles, and chemical-resistant clothing and shoes are used to minimize risks when handling butyric acid.
Inhalation of butyric acid may result in soreness of throat, coughing, a burning sensation and laboured breathing. Ingestion of the acid may result in abdominal pain, shock, and collapse. Physical exposure to the acid may result in pain, blistering and skin burns, while exposure to the eyes may result in pain, severe deep burns and loss of vision.
잠재적 노출
In manufacture of butyrate esters, some of which go into artificial flavoring. Incompatibilities: May form explosive mixture with air. Incompatible with sulfuric acid, caustics, ammonia, aliphatic amines; isocyanates, strong oxidizers; alkylene oxides; epichlorohydrin운송 방법
UN2820 Butyric acid, Hazard class: 8; Labels: 8—Corrosive material. UN2529 Isobutyric acid, Hazard Class: 3; Labels: 3—Flammable liquid, 8—Corrosive materialPurification Methods
Distil the acid, them mix it with KMnO4 (20g/L), and fractionally redistil, discarding the first third of the distillate [Vogel J Chem Soc 1814 1948]. [Beilstein 2 IV 779.]폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.부탄 산 준비 용품 및 원자재
원자재
Concentrated hydrochloric acid
코발트(II) 아세테이트
산소, 냉각된 액체
tert-부틸알코올
BUTTER
n-뷰탈알데히드
1-펜타놀
질산
바닐린
당밀
Manganese triacetate dihydrate
준비 용품
부틸산이소아밀
Progabide
무수부틸산
FEMA 3332
(+/-)-펜토텐 산, 칼슘 염 모노수화물
부틸산에틸
disodium 3-[[4'-[(6-amino-1-hydroxy-3-sulphonato-2-naphthyl)azo]-3,3'-dimethoxy[1,1'-biphenyl]-4-yl]azo]-4-hydroxynaphthalene-1-sulphonate
L-(+)-아이소루신
Reactive Red Brown K-B3r
2-에틸-1,3-시클로펜탄디온
1,4-Bis(4-cyanostyryl)benzene
이소낙산
1-OCTEN-3-YL BUTYRATE
디프로필 케톤
Vat Orange 9
CIS-3-HEXENYL BUTYRATE
ALPHA-KETOBUTYRIC ACID SODIUM SALT
FEMA 2686
Leather Black
부티릴클로라이드
벤질 디메틸 카르비닐 부티레이트
Direct Blue 71
FEMA 2368
알릴 뷰틸산
펜발러레이트
α-브로모부틸산
Phenethyl butyrate
아세트산(빙초산)
Butyramide
Reactive Orange 1
CYCLOHEXYL BUTYRATE
부탄 산 공급 업체
글로벌( 607)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21666 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 |
figo.gao@foxmail.com | China | 7621 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2966 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |