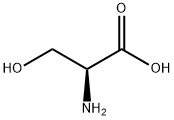
L-(-)-세린
|
|
L-(-)-세린 속성
- 녹는점
- 222 °C (dec.) (lit.)
- 알파
- 15.2 º (c=10, 2N HCl)
- 끓는 점
- 197.09°C (rough estimate)
- 밀도
- 1.6
- 굴절률
- 1.4368 (estimate)
- 인화점
- 150°C
- 저장 조건
- 2-8°C
- 용해도
- H2O: 50 mg/mL
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 2.19(at 25℃)
- 색상
- 하얀색
- 수소이온지수(pH)
- 5-6 (100g/l, H2O, 20℃)
- 냄새
- 백색결정분말, 무취, 단맛
- optical activity
- [α]20/D +13.5±0.5°, c = 5% in 5 M HCl
- 수용성
- 250 g/L (20 ºC)
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.05
λ: 280 nm Amax: 0.05
- Merck
- 14,8460
- 승화점
- 150 ºC
- BRN
- 1721404
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- InChIKey
- MTCFGRXMJLQNBG-REOHCLBHSA-N
- LogP
- -1.580
- CAS 데이터베이스
- 56-45-1(CAS DataBase Reference)
- NIST
- L-Serine(56-45-1)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 24/25-36-26 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | VT8100000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 29225000 | ||
| 유해 물질 데이터 | 56-45-1(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-01418 |
L-(-)-세린 C화학적 특성, 용도, 생산
순도시험
(1) 비선광도 : 이 품목을 미리 건조한 다음 10g을 정밀히 달아 2N 염산을 가하여 녹인 다음 100mL로 하여 이 액의 선광도를 측정할 때, 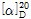 =+13.6~+16.0°이어야 한다.
=+13.6~+16.0°이어야 한다.
(2) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 염화물 : 이 품목 0.07g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.2mL에 대응하는 양 이하이어야 한다(0.1% 이하).
확인시험
(1) 이 품목의 수용액(1→1,000) 5mL에 닌히드린용액(0.2→100) 1mL를 가한 후 수욕조에 3분간 가열하면 적자~자색을 나타낸다.
(2) 이 품목 약 0.5g을 물 10mL에 녹이고 과요오드산 0.2g을 가하여 가열하면 포름알데히드 냄새가 난다.
정량법
이 품목 약 0.2g을 정밀히 달아 개미산 3mL 및 빙초산 50mL를 가하여 녹인 다음 0.1N 과염소산용액으로 적정한다(지시약 : 크리스탈바이올렛․빙초산시액 2방울). 종말점은 액의 색이 청녹색으로 변하는 점이다. 따로 같은 방법으로 공시험을 한다.
0.1N 과염소산용액 1mL = 10.51mg C3H7NO3
강열잔류물
이 품목의 강열잔류물은 0.1% 이하이어야 한다.
개요
Serine (abbreviated as Ser or S) is an amino acid with the formula HO2CCH(NH2)CH2OH. It is one of the proteinogenic amino acids. Its codons in the genetic code are UCU, UCC, UCA, UCG, AGU and AGC. By virtue of the hydroxyl group, serine is classified as a polar amino acid.L-serine is an amino acid essential for the synthesis of phosphatidylserine, which is a component of the membrane of brain cells (i.e., neurons). It can be produced in the body, including the brain, but an external supply from the diet is essential in maintaining necessary levels. Although preclinical studies suggest L-serine may inhibit inflammation in the brain, levels of L-serine in humans do not appear to be associated with dementia or cognitive decline. Because L-serine is a naturally occurring amino acid, supplementation is likely safe in moderation.
화학적 성질
L-Serine is a white crystal or crystalline powder, slightly sweet taste, soluble in water and acid, insoluble in alcohol and ether. From soybeans, wine fermentation agent, dairy products, eggs, fish, lactalbumin, meat, nuts, seafood, whey, and whole grains to get. Serine is also used as a dietary supplement where it can aid improved memory function and in personal care products where it helps with the production of new skin cells.용도
L-Serine has been used in the preparation of Tris-BSAN buffer for homogenization. It has also been used for quantitative analysis of excretion of polypeptides in normal urine.생산 방법
Industrially , L - serine is produced by fermentation, with an estimated 100 - 1000 tonnes per year produced . In the laboratory, racemic serine can be prepared from methyl acrylate via several steps.정의
ChEBI: L-serine is the L-enantiomer of serine. It has a role as a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a serine family amino acid, a proteinogenic amino acid, a L-alpha-amino acid and a serine. It is a conjugate base of a L-serinium. It is a conjugate acid of a L-serinate. It is an enantiomer of a D-serine. It is a tautomer of a L-serine zwitterion.일반 설명
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.생물학적 활성
L-Serine is an endogenous agonist at the inhibitory glycine receptor.L-Serine is a non-essential amino acid that acts as a precursor for nucleotide synthesis. It has roles in the development and function of the central nervous system. L-Serine also has a role in cellular proliferation.
Precursor of glycine by serine hydroxymethyltransferase.
Safety
L-serine is generally safe to take as an oral dietary supplement. In a phase 1 safety trial in 20 ALS patients, L-serine treatment (0.5-15.0 g, twice daily) for 6 months was generally well-tolerated and appeared to be safe. Two patients withdrew from the trial due to gastrointestinal side effects but no other adverse events or changes in routine blood tests measures were observed.Purification Methods
A likely impurity is glycine. Crystallise L-serine from H2O by adding 4volumes of EtOH. Dry and store it in a desiccator. It sublimes at 160-170o/0.3mm with 99.7% recovery, and unracemised [Gross & Gradsky J AmL-(-)-세린 준비 용품 및 원자재
원자재
Acid off
암모니아수
Boc-D-Tyr-OH
포름산에틸
아미노아실라제
AMberlite 732
Pure water
Α-protein
ANION EXCHANGE RESIN 717
732 resin
클로로아세틸 클로라이드
Ion exchange resin column
초산에틸
DL-세린
에틸히푸레이트
염화 폴리비닐
준비 용품
L-(-)-세린 공급 업체
글로벌( 874)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Apeloa Pharmaceutical Co., Ltd. | +86-0571-87635730 +8615858229168 |
chem1022@hengdian-group.com | China | 1478 | 58 |
| Suzhou Actchem Co., Ltd. | +8618762124502 |
actchem@qq.com | China | 27 | 58 |
| ChemExpress | +86-021-58950125 +86-021-58950125; |
info@chemexpress.com | China | 598 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12465 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5895 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18628 | 58 |
| Taizhou Tianhong Biochemistry Technology Co., Ltd. | 0523-86132544 |
sales@thbiochem.com | CHINA | 305 | 60 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 299 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21666 | 55 |