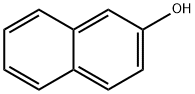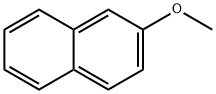
2-Methoxynaphthalene synthesis
- Product Name:2-Methoxynaphthalene
- CAS Number:93-04-9
- Molecular formula:C11H10O
- Molecular Weight:158.2
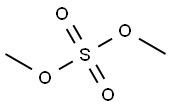
Principle: Phenols can be methylated to give methyl ethers. Methylation can be done either by using diazomethane or dimethyl sulphate in alkaline medium.
Reaction:
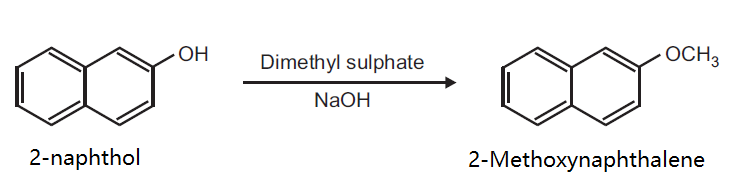
Procedure: Take 0.5 g 2-naphthol and 0.2 g NaOH in 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Cool the solution (10-15°C) and then add 0.35 ml dimethyl sulphate drop wise. After the addition is over, warm the mixture for one hour at 70-80°C and then cool. Filter the product and wash it with 10% sodium hydroxide solution and then with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. Filter the white crystals of the product. Dry and record the melting point and TLC (using toluene as solvent).
Yield:93-04-9 99%
Reaction Conditions:
at 180; for 1 h;
Steps:
2-Methoxynaphthalene (8)
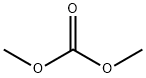
General procedure: General procedure for the alkylation of phenols with dimethyl carbonate. A 17-mL stainless steel high-pressure micro reactor was charged with 3 mmol of Mn2(CO)10, W(CO)6, or Co2(CO)8, 100 mmol of the corresponding phenol, and 300 mmol of dimethyl carbonate, and the reactor was hermetically closed and heated for 1 h at 180°C. The reactor was then cooled to room temperature and opened, and the mixture was filtered through a layer of alumina. Unreacted dimethyl carbonate was distilled off, and the residue was distilled under atmospheric or reduced pressure or recrystallized from ethanol. 2-Methoxynaphthalene (8). Yield 99%, mp 73-74°C (from EtOH). 13C NMR spectrum, δC, ppm: 55.50 (OCH3), 106.69 (C1), 119.15 (C3), 123.72 (C6), 124.75 (C8), 125.68 (C5), 127.01 (C7), 127.55 (C4), 130.12 (C10), 135.77 (C9), 157.72 (C2). Found, %: C 83.42; H 6.34. C11H10O. Calculated, %: C 83.51; H 6.37.
References:
Khusnutdinov;Shchadneva;Mayakova, Yu. Yu. [Russian Journal of Organic Chemistry,2015,vol. 51,# 3,p. 330 - 334][Methylation of Phenol and Its Derivatives with Dimethyl Carbonate in the Presence of Mn2(CO)10, W(CO)6, and Co2(CO)8,2015,vol. 51,# 3,p. 330 - 334,5]
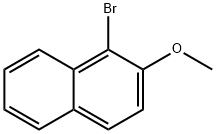
3401-47-6
174 suppliers
$62.00/5G

93-04-9
475 suppliers
$13.00/25g