Thiamin Chemische Eigenschaften,Einsatz,Produktion Methoden
Allgemeine Beschreibung
Thiamine, the preferred name for vitamin B
1, holds a prominent place in the history of vitamin discovery because beriberi, the disease resulting from insufficient thiamine intake, was one of the earliest recognized deficiency diseases.
Biologische Aktivität
Some earlier designations for this substance included aneurin, antineuritic factor, antiberiberi factor, and oryzamin. Thiamine is metabolically active as thiamine pyrophosphate (TPP).
TPP functions as a coenzyme which participates in decarboxylation of α-keto acids. Dehydrogenation and decarboxylation must precede the formation of “active acetate” in the initial reaction of the TCA cycle (citric acid cycle):
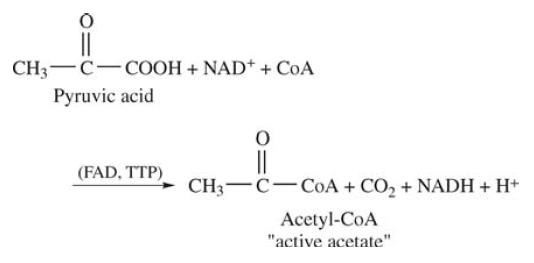
This reaction is a good example of the interrelationship of vitamin B coenzymes. Four vitamin coenzymes are necessary for this one reaction: (1) thiamine (in TPP) for decarboxylation; (2) nicotinic acid in nicotinamide adenine dinucleotide (NAD); (3) riboflavin in flavin adenine dinucleotide (FAD); and (4) pantothenic acid in coenzyme A (CoA) for activation of the acetate fragment.
Clinical Use
Thiamine chloride, as the base or as the hydrochloride salt, is indicatedin the treatment or prophylaxis of known or suspected thiaminedeficiencies. Severe thiamine deficiency is calledberiberi, which is very rare in developed countries. The mostlikely cause of thiamine deficiency in the United States is theresult of chronic alcoholism, which leads to multiple vitamindeficiencies as a result of poor dietary intake. The major organsaffected are the nervous system (dry beriberi), whichmanifests as neurological damage, the cardiovascular system(wet beriberi), which manifests as heart failure and edema, andthe gastrointestinal tract. Thiamine administration reverses thegastrointestinal, cardiovascular, and neurological symptoms;however, if the deficiency has been severe or of prolonged duration, the neurological damage may be permanent.
Sicherheitsprofil
Poison by
subcutaneous and intravenous routes. An
experimental teratogen. Experimental
reproductive effects. When heated to
decomposition it emits very toxic fumes of
NOx, SOx, and Cl-.
Thiamin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

