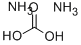
Ammonium carbonate
- CAS No.
- 506-87-6
- Chemical Name:
- Ammonium carbonate
- Synonyms
- Glucitol;crystalammonia;hartshorn;HARTSHORN SALT;BROMOCRESOL GRE;ammoniumcarbonat;SALT OF HARTSHORN;AMMONIUM CARBONATE;diammoniumcarbonate;AMMONIU BICARBONATE
- CBNumber:
- CB9393644
- Molecular Formula:
- CH8N2O3
- Molecular Weight:
- 96.09
- MDL Number:
- MFCD00010890
- MOL File:
- 506-87-6.mol
- MSDS File:
- SDS
| Melting point | 58 °C |
|---|---|
| Boiling point | 179.58°C (rough estimate) |
| Density | 1.5 |
| vapor density | 2.7 (vs air) |
| vapor pressure | 760 mm Hg @ 600C |
| refractive index | 1.4616 (estimate) |
| storage temp. | Store at RT. |
| solubility | H2O: 0.1 g/mL at 25 °C, clear, colorless |
| form | Solid |
| color | White to yellow |
| Odor | strong odor of NH3 |
| PH Range | 9 |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,508 |
| BRN | 3627235 |
| LogP | -0.809 (est) |
| CAS DataBase Reference | 506-87-6(CAS DataBase Reference) |
| FDA 21 CFR | 184.1137; 582.1137 |
| EWG's Food Scores | 1 |
| FDA UNII | PDP691CN28 |
| EPA Substance Registry System | Ammonium carbonate (506-87-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-52/53 | |||||||||
| Safety Statements | 24/25 | |||||||||
| RIDADR | UN 9084 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BP1925000 | |||||||||
| F | 13 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28369940 | |||||||||
| Toxicity | LD50 ivn-mus: 96 mg/kg AJVRAH 29,897,68 | |||||||||
| NFPA 704 |
|
Ammonium carbonate price More Price(58)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 11204 | Ammonium carbonate puriss., meets analytical specification of NF, Ph. Franc., FCC | 506-87-6 | 1kg | $78.1 | 2024-03-01 | Buy |
| Alfa Aesar | 012980 | Ammonium carbonate, Puratronic?, 99.999% (metals basis) | 506-87-6 | 50g | $153 | 2024-03-01 | Buy |
| Alfa Aesar | 012980 | Ammonium carbonate, Puratronic?, 99.999% (metals basis) | 506-87-6 | 10g | $51 | 2023-06-20 | Buy |
| Sigma-Aldrich | 207861 | Ammonium carbonate ACS reagent, ≥30.0% NH3 basis | 506-87-6 | 25g | $28.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 207861 | Ammonium carbonate ACS reagent, ≥30.0% NH3 basis | 506-87-6 | 100g | $57.7 | 2024-03-01 | Buy |
Ammonium carbonate Chemical Properties,Uses,Production
Outline
Ammonium carbonate is positive ammonium salt of carbonic acid normal salt, the formula is (NH4)2CO3. Pure product is colorless or white cubic crystal or powder, it has strong smell of ammonia. Industrial product is complex salt of ammonium bicarbonate and ammonium carbamate, it is white, flaky or small block of solid product crushed form. It is often with a molecular crystal water, it is hygroscopic, soluble in water, it can decompose in case of hot water. It is insoluble in ethanol and carbon disulfide. Ammonium carbonate can rapidly decompose into ammonia, carbon dioxide and water at 58℃. Ammonium carbonate can be obtained by ammonia introduces into solution of sodium carbonate with half times, the solution crystallizes at 30°C. It gradually loses ammonia to form ammonium bicarbonate in air.
Solubility in water (g/100ml)
The grams which dissolve per 100 ml of water:100g/20 ℃.
Related reactions of the formula
At room temperature for significant decomposition: (NH4) 2CO3 → 2NH3 + CO2 + H2O
At low temperature and a certain pressure, carbon dioxide and water with an excess of ammonia, ammonium carbonate can be obtained: 2NH3 + CO2 + H2O → (NH4) 2CO3
Ammonium sulfate and calcium carbonate suspension under heating to generate ammonium carbonate: (NH4) 2SO4 + CaCO3 → (NH4) 2CO3 + CaSO4
Urea in aqueous solution will gradually react with water to form ammonium carbonate: CO (NH2) 2 + 2H2O → (NH4) 2CO3
Toxcity
If it splashes into the eye accidentally, rinse immediately with plenty of water. It has stimulating effect on the skin. It should pay attention to dust prevention and dust extraction, respiratory protection, skin protection.
Chemical properties
It is matte orthorhombic crystalline powder. It has strong ammonia odor. It usually can not get anhydrous salt, industrial salt is actually a complex of ammonium bicarbonate and ammonium carbamate. The amount of ammonia is 31%, The amount of carbon dioxide is 56%. It is soluble in water, insoluble in ethanol, carbon disulfide, and concentrated ammonia. It is unstable in the air, it will gradually become ammonium bicarbonate and ammonium carbamate. When be dried at 58℃, it can easily decompose, release ammonia and carbon dioxide. Aqueous solution begins to decompose at 70℃. It is unstable for light and heat. It has slightly hygroscopic.
Uses
It is used as raw material for baking powder, various ammonium salts, buffer agent, auxiliaries, fertilizer and analytical reagent. Edible ammonium carbonate is used as buffer, neutralizing agent, leavening agent, fermentation promoter (manufacture of wine).
It is used for fire fighting, detergents, and used in medicine, rubber, and other industrial fermentation.
The above information is edited by the chemicalbook of Wang Xiaodong.
Production method
Carbonization method: Carbon dioxide, ammonia and steam synthesized directly sodium carbonate, it passes through the cooling chamber, uses water to direct cooling, and then it is refined to obtain ammonium carbonate products.
2NH3 + CO2 + H2O → (NH4) 2CO3
Category
Toxic substances.
Toxicity grading
highly toxic.
Acute toxicity
Intravenous-Mouse LD50: 96 mg/kg; Intravenous-Dogs LDL0: 200 mg/kg.
Flammability hazard characteristics
It can produce toxic fumes of nitrogen oxides and ammonia at high temperature.
Storage characteristics
Treasury ventilation low-temperature drying.
Extinguishing agent
Dry powder, foam, sand, carbon dioxide, water mist.
Chemical Properties
Anunonium carbonate is a white water-soluble, volatile solid prepared by reaction of NH4OH and CO2 and crystallizing from dilute alcohol. Ammonium carbonate loses NH3,CO2, and H20 at ordinary temperatures, and rapidly at 58°C.
Chemical Properties
Ammonium carbonate is a colorless crystal or white lumpy powder with a strong ammonia odor. The odor specific gravity (gas)52.7; Threshold is ,5 ppm as ammonia gas.
Physical properties
Colorless or translucent hard crystalline mass or white cubic crystals or powder; sharp taste; odor of ammonia; decomposes at 58°C; slow decomposition at ambient temperatures; readily dissolves in cold water; decomposes in hot water; insoluble in liquid ammonia, alcohol and carbon disulfide.
Uses
Pharmaceutic aid (source of ammonia).
Uses
Ammonium carbonate is widely utilized as a leavening agent in lebkuchen, cookies and flat biscuits. It finds an important application as an emetic and an active ingredient in some cough syrups. It is the main component in smelling salts and an active ingredient in some smokeless tobacco products, shampoos and dyes used in textile industries. It is an important predecessor to the modern leavening agent?s baking soda and baking powder. It serves as a foaming agent for the production of expanded material. It is also used as a reagent for analytical purposes in the chemical industry.
Uses
Ammonium Carbonate is a dough strengthener, a leavening agent, a ph control agent, and a texturizer. it is prepared by the sublima- tion of a mixture of ammonium sulfate and calcium carbonate, and occurs as a white powder or a hard, white translucent mass.
Definition
A mixture of ammonium acid carbonate and ammo- nium carbamate.
Preparation
Ammonium carbonate is obtained by passing carbon dioxide into aqueous ammonia solution in a column or tower. Ammonia, carbon dioxide and water vapor are distilled and the vapors condensed into a solid crystalline mass. It also may be prepared by subliming a mixture of ammonium sulfate and calcium carbonate.
Definition
ammonium carbonate: A colourlessor white crystalline solid,(NH4)2CO3, usually encountered asthe monohydrate. It is very soluble incold water. The compound decomposesslowly to give ammonia, water,and carbon dioxide. Commercial ‘ammoniumcarbonate’ is a double saltof ammonium hydrogencarbonateand ammonium aminomethanoate(carbamate), NH4HCO3.NH2COONH4.This material is manufactured byheating a mixture of ammoniumchloride and calcium carbonate andrecovering the product as a sublimedsolid. It readily releases ammoniaand is the basis of sal volatile. It isalso used in dyeing and wool preparationand in baking powders.
General Description
A colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing.
Air & Water Reactions
Water soluble.
Reactivity Profile
Ammonium carbonate decomposes when heated to give gaseous ammonia and gaseous carbon dioxide. Reaction is non-explosvie. Causes decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].
Hazard
Evolves irritating fumes when heated.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion may cause gastric irritation. Contact with eyes or skin causes irritation.
Agricultural Uses
Ammonium carbonate, (NH4)2CO3, is an intermediate product formed during the synthesis of urea. Ammonium carbonate on decomposition yields urea and water.
Safety Profile
Poison by subcutaneous and intravenous routes. When heated to decomposition it emits toxic fumes of NO, and NH3.
Potential Exposure
It is used in dyeing, tanning, medicines, fire extinguishers; to make casein glue; ammonia salts; and baking powders. A laboratory reagent.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Acids, acid salts; salts of iron and zinc, alkaloids, calomel and tartar emetic. Keep cool, below 38 C. Contact with inorganic acids may form CO2, heat, and dangerous spattering.
Waste Disposal
Slowly deposit in a large container of water. Add excess amounts of soda ash and let stand for 24 hours. Decant to another container, neutralize with hydrochloric acid, and drain with an excess of water. Ship to landfill.
Ammonium carbonate Preparation Products And Raw materials
Raw materials
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5895 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Jimi Trading Co., Ltd. | +86 319 5273535 | bestoneforyou@sina.com | CHINA | 288 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
View Lastest Price from Ammonium carbonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | Ammonium Carbonate
506-87-6
|
US $0.70 / kg | 10kg | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-08-15 | Ammonium carbonate
506-87-6
|
US $0.60 / KG | 25KG | 98% | 2000 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Ammonium carbonate
506-87-6
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Ammonium Carbonate
506-87-6
- US $0.70 / kg
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Ammonium carbonate
506-87-6
- US $0.60 / KG
- 98%
- Hebei Yanxi Chemical Co., Ltd.
-

- Ammonium carbonate
506-87-6
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.