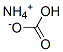
Ammonium bicarbonate
- CAS No.
- 1066-33-7
- Chemical Name:
- Ammonium bicarbonate
- Synonyms
- AMMONIUM HYDROGEN CARBONATE;acidammoniumcarbonate;AMMONIUM ACID CARBONATE;2,6'-TDI, Oekanal;Ammonium Hydrogen;ammonium bicarbonat;AMMONIUM BICARBONATE;Ammonium dicarbonate;AmmoniumBicarbonateBp;AmmoniumBicarbonateAr
- CBNumber:
- CB9354407
- Molecular Formula:
- CH5NO3
- Molecular Weight:
- 79.06
- MDL Number:
- MFCD00012138
- MOL File:
- 1066-33-7.mol
- MSDS File:
- SDS
| Melting point | 105 °C |
|---|---|
| Boiling point | 143.04°C (rough estimate) |
| Density | 1,586 g/cm3 |
| vapor density | 2.7 (vs air) |
| vapor pressure | 67 hPa (20 °C) |
| refractive index | 1.4164 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White |
| Odor | faint ammonia odor |
| PH | 7.0-8.5 (25℃, 1M in H2O) |
| Water Solubility | 220 g/L (20 ºC) |
| λmax |
λ: 260 nm Amax: ≤0.030 λ: 280 nm Amax: ≤0.020 |
| Merck | 14,497 |
| BRN | 4329606 |
| Stability | Stable. Incompatible with strong acids, alkali metals. |
| LogP | -0.809 (est) |
| Dissociation constant | 6.49 at 20℃ |
| FDA 21 CFR | 184.1135; 582.1135 |
| Substances Added to Food (formerly EAFUS) | AMMONIUM BICARBONATE |
| SCOGS (Select Committee on GRAS Substances) | Ammonium bicarbonate |
| CAS DataBase Reference | 1066-33-7(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 45JP4345C9 |
| EPA Substance Registry System | Ammonium bicarbonate (1066-33-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 22 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BO8600000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2836 99 17 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1576 mg/kg | |||||||||
| NFPA 704 |
|
Ammonium bicarbonate price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 5.43835 | Ammonium hydrogen carbonate for HPLC LiChropur? | 1066-33-7 | 100g | $93.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.43835 | Ammonium hydrogen carbonate for HPLC LiChropur? | 1066-33-7 | 250g | $210 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.33005 | Ammonium hydrogen carbonate for LC-MS LiChropur? | 1066-33-7 | 50g | $107 | 2024-03-01 | Buy |
| Sigma-Aldrich | 11213 | Ammonium bicarbonate puriss., meets analytical specification of Ph.Eur., BP, E 503, 99-101% | 1066-33-7 | 1kg | $113 | 2024-03-01 | Buy |
| Sigma-Aldrich | 11213 | Ammonium bicarbonate puriss., meets analytical specification of Ph.Eur., BP, E 503, 99-101% | 1066-33-7 | 6x1kg | $193 | 2024-03-01 | Buy |
Ammonium bicarbonate Chemical Properties,Uses,Production
description
Ammonium bicarbonate(1066-33-7) is a commonly used reagent for industrial and research procedures. Ammonium bicarbonate is volatile in solution and releases ammonia and CO2. This property makes ammonium bicarbonate a good buffer for such applications as lyophilization and matrix assisted laser desorption. Ammonium bicarbonate is also utilized for the in-gel digestion of proteins by trypsin and in the MALDI mass spectrometric analysis of proteins.

Ammonium bicarbonate is used as a baking powder, in some food processing applications, in cough syrups and as antacid. It also has uses as a fertilizer, pH buffer, and reagent in chemical laboratories. In the industry, it is used in the manufacture of dyes, pharmaceuticals, catalysts, ceramics, fire-retardants, plastics and other products.
Nitrogen fertilizer
Ammonium bicarbonate is mainly used as fertilizers. After being applied to the soil, the ammonium ion (NH4 +) contained in ammonium bicarbonate can be absorbed by soil colloid or lattice-fixed or transformed into nitrate nitrogen. After being absorbed by plants, there are no accessory constituents remaining in the soil with a small impact on the soil pH. It is applicable to all kinds of soil and crops, soil without leaving any harmful substance residue for the soil and crops. It is quick-acting nitrogen fertilizer and can be subject to long-term usage.
In order to prevent the loss of fertilizer efficacy due to ammonia volatilization and the burning of the crop stems and leaves, we can apply deep placement and cover soil. It can be used as basic fertilizer for topdressing, but not suitable to be used as seed manure. When being used for topdressing, we should prevent the drop of ammonium bicarbonate onto the plant, to avoid ammonia hazards. The shortcoming of ammonium bicarbonate as a fertilizer lies in its chemical instability. After the addition of crystalline modifier, the crystal of ammonium bicarbonate is enlarged and the water content is reduced, reducing the phenomenon of easily subjecting to decomposition and agglomeration. Ammonium bicarbonate is one of nitrogen fertilizer industrial products, being the major varieties of small nitrogenous fertilizer plants in China, being one of the purification products of coke oven in the coking plant. Coking plant takes concentrated ammonia as raw materials for reaction with carbon dioxide to generate ammonium bicarbonate crystals with centrifugal filtering to obtain ammonium bicarbonate products.
Ammonium bicarbonate is easy to be subject to decomposition. It is appropriate to be packed with the combination of inner plastic film and external plastic bag or 3-layers of kraft paper sacks, both need to be sealed and stored in a warehouse of being cool, low-temperature, dry and ventilated to prevent moisture, rain and sun.
Chemical properties
Ammonium bicarbonate appears as white monoclinic or orthorhombic crystals. It is soluble in water, but insoluble in ethanol, carbon disulfide and concentrated ammonia.
It dissolves in water to give a mildly alkaline solution. It is insoluble in most organic solvents. While it is stable at room temperature (25 °C), it decomposes at temperatures above 36 °C to form ammonia, carbon dioxide, and water in an endothermic reaction (absorbs energy for the reaction from the surroundings).
NH4HCO3 → NH3 + CO2 + H2O
Ammonium bicarbonate reacts with acids to produce carbon dioxide, and reacts with bases to produce ammonia.
Uses
1. Ammonium bicarbonate(1066-33-7) is used as nitrogen fertilizer, being applicable to a variety of soils, can simultaneously provide the ammonium nitrogen and carbon dioxide demanded by crop growth. However, it contains low nitrogen content and is also easy to caking.
2. It can be used as analytical reagent as well as being used in synthesizing ammonium salt and fabric degreasing.
3. It can promote crop growth and photosynthesis; trigger seedlings and the growth of leaves. It can be used as topdressing as well as being directly applied as ground fertilizer as food leavening agent and bulking agent.
4. Ammonium bicarbonate can be used as a senior food fermentation agent. Its combination with sodium bicarbonate can be used as the raw materials of leavening agent such as bread, biscuits and pancakes. It can also be used as raw material of foam powder juice, as well as being used for the blanching of green vegetables and bamboo shoots. Moreover, it can be used as medicine and reagents.
5. Alkali; leavening agent; buffer; aerating agent.
Its combination with sodium bicarbonate can be used as the raw materials of leavening agent such as bread, biscuits and pancakes. Baking powder also takes this product as the main ingredient, together with the acidic substances. It can also be used as raw material of foam powder juice. The dosage of the blanching of green vegetables and bamboo shoots should be 0.1% to 0.3%.
6. It can be used as analytical reagent; used for ammonium salt synthesis. Pharmaceuticals; baking powder; dyeing; It can be used for fabric degreasing. It can also be used as foamed plastics.
Production method
Send the compressed carbon dioxide into the concentrated ammonia, and place it under the carbon dioxide pressure; simultaneously apply cooling; precipitate out the crystal; followed by centrifugal separation and dehydration to obtain the final product. Upon refining, dissolve it in water, and add ethanol to re-crystallize it.
Carbonization method: after the ammonia is absorbed by water; apply carbon dioxide for carbonization, followed by separation and drying to produce ammonium bicarbonate.
NH3 + CO2 + H2O → NH4HCO3
To the carbon dioxide gas originated from the lime kiln and be subject to cleaning and washing, send the ammonia to saturation, followed by centrifugal separation and hot air drying to obtain the finished products.
NH3 + CO2 + H2O → NH4HCO3
Chemical Properties
Ammonium bicarbonate is a white crystalline solid with a faint ammonia odor and soluble in water but insoluble in alcohol and acetone. It decomposes above 35℃ to ammonia, carbon dioxide and water vapor, releasing irritant fumes. Only 30% of the applied nitrogen of this fertilizer is recovered by plants owing to the unstable nature of ammonium bicarbonate. that forms by the reaction of anunonium hydroxide and excess CO2.
Physical properties
White crystalline solid; prismatic crystal; faint odor of ammonia; stable at ambient temperature but decomposes on heating at 60°C; melts at 107.5°C on very rapid heating; density 1.586 g/cm3; vapor pressure 435 torr at 25°C; readily dissolves in water (21.6g/100g at 20°C, and 36.6g/100g at 40°C).
Uses
Ammonium Bicarbonate is a dough strengthener, a leavening agent, a ph control agent, and a texturizer. prepared by reacting gaseous carbon dioxide with aqueous ammonia. crystals of ammo- nium bicarbonate are precipitated from solution and subsequently washed and dried. Also known as hartshorn and rock ammonia, ammonium bicarbonate is soluble in water but decomposes when heated. It was used in place of ammonia when making ammonia-ripened gelatin emulsions.
Uses
Ammonium bicarbonate is a commonly used reagent for industrial and research procedures. It acts a good buffer in lyophilization and matrix assisted laser desorption. It is also utilized for the in-gel digestion of proteins by trypsin and in the MALDI mass spectrometric analysis of proteins. It is also used to make other ammonium compounds, in food processing, and for other uses. Ammonium bicarbonate can be used to study biological buffers. It is also used in a study that demonstrated that ammonium bicarbonate salts, which can be regenerated using low-temperature waste heat, can also produce sufficient voltage for hydrogen gas generation in a microbial reverse-electrodialysis electrolysis cells. It has also been used in a study that developed a fast and sensitive method for the simultaneous determination of Sudan dyes in food samples using partial filling micellar electrokinectic chromatography-mass spectrometry.
Definition
ChEBI: Ammonium bicarbonate is an organooxygen compound. It is a buffer applications such as lyophilization and matrix assisted laser desorption.
General Description
A white crystalline solid having the odor of ammonia. Soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in food processing, and for other uses.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Heat > 36°C ( produces ammonia and carbon dioxide); strong acids and strong bases (CO2 and NH3) [Handling Chemicals Safely 1980 p. 141].
Hazard
Evolves irritating fumes on heating to 35C.
Health Hazard
Inhalation may cause respiratory irritation. Ingestion could be harmful. Contact with eyes or skin causes irritation.
Breathing Ammonium Bicarbonate can irritate the nose, throat, and lungs causing coughing, wheezing, and/or shortness of breath. No risks to humans are expected from approved uses of ammonium bicarbonate as a pesticide active ingredient. The substance is approved as a food additive by the Food and Drug Administration (FDA) and as an inert pesticide ingredient by EPA. No toxic endpoints have been identified.
Agricultural Uses
Ammonium hydrogen carbonate is another name for ammonium bicarbonate (NH4CO3), It is a low nitrogen containing fertilizer (17% N), used largely in China. It is produced by heating ammonium hydroxide with excess carbon dioxide, followed by evaporation of water.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of NO, and NH3
Potential Exposure
It is used in leavening for some baked goods; in baking powders and fire extinguishers; to make dyes and pigments; in the manufacture of porous plastics; and as an expectorant.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Contact with strong caustics, such as potassium hydroxide or sodium hydroxide will cause the release of ammonia gas. Decomposes as temperature rises >35 C.
Waste Disposal
May be buried in a chemical waste landfill. If neutralized ammonium bicarbonate is amenable to treatment at a municipal sewage treatment plant.
Ammonium bicarbonate Preparation Products And Raw materials
Raw materials
1of5
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1009 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3878 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5895 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 | admin@juchuangchem.com | China | 387 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18032476855 +86-18032476855 | admin@hblongbang.com | China | 942 | 58 |
| Hebei Shengyang Water Conservancy Engineering Co., Ltd. | +8615373025980 | clara@hbshengyang.com | China | 874 | 58 |
Related articles
- Can Ammonium bicarbonate be used as a food additive?
- Ammonium bicarbonate is a clear white crystalline solid with a faint ammonia smell. It slowly degrades the environment into am....
- Apr 9,2024
Related Qustion
- Q:What does Ammonium bicarbonate do?
- A:Ammonium bicarbonate is an important bulking agent in the biscuit and cracker industry, and is also used by bakers in some str....
- Dec 22,2023
View Lastest Price from Ammonium bicarbonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-26 | Ammonium bicarbonate
1066-33-7
|
US $10.60 / KG | 1KG | 99% | 5000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-26 | Imidodisulfurylfluoride
1066-33-7
|
US $999.00-900.00 / ton | 0.0010000000474974513ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-26 | Ammonium bicarbonate
1066-33-7
|
US $0.00-0.00 / TON | 1TON | 99.5% | 1000000 | Hebei Jingbo New Material Technology Co., Ltd |
-

- Ammonium bicarbonate
1066-33-7
- US $10.60 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Imidodisulfurylfluoride
1066-33-7
- US $999.00-900.00 / ton
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Ammonium bicarbonate
1066-33-7
- US $0.00-0.00 / TON
- 99.5%
- Hebei Jingbo New Material Technology Co., Ltd