비페닐 C화학적 특성, 용도, 생산
개요
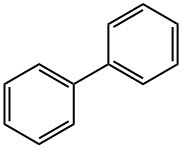
Biphenyl, also called diphenyl, consists of two benzene rings joined by a single bond. It exists as colorless to yellowish crystals, has a distinctive odor, and occurs naturally in oil, natural gas, and coal tar.
화학적 성질
1,1'-Biphenyl is a clear colorless liquid with a pleasant odor and stable organic compound. It is combustible at high temperatures, producing carbon dioxide and water when combustion is complete. Partial combustion produces carbon monoxide, smoke, soot, and low molecular weight hydrocarbons. It is used extensively in the production of heat-transfer fl uids, for example, as an intermediate for polychlorinated biphenyls, and dye carriers for textile dyeing. It is also used as a mold retardant in citrus fruit wrappers, in the formation of plastics, optical brighteners, and hydraulic fluids.
물리적 성질
White scales with a pleasant but peculiar odor. Odor threshold concentration is 0.83 ppb (quoted,
Amoore and Hautala, 1983).
출처
Reported found in coal tar, bilberry, carrots, peas, potato, bell pepper, rum, cocoa, tomato, coffee, roasted
peanuts, olive (Olea europae), buckwheat and tamarind (Tamarindus indica L).
용도
Biphenyl is used as an antifungal agent to preserve citrus fruit, in citrus wrappers to retard mold growth, in heat transfer fluids, in dye carriers for textiles and copying paper, as a solvent in pharmaceutical production, in optical brighteners, and as an intermediate for the production of a wide range of organic compounds.
제조 방법
By thermal dehydrogenation of benzene.
정의
ChEBI: A benzenoid aromatic compound that consists of two benzene rings connected by a single covalent bond. Biphenyl occurs naturally in coal tar, crude oil, and natural gas. Formerly used as a fungicide for citrus crops.
일반 설명
A clear colorless liquid with a pleasant odor. Flash point 180°F. Insoluble in water. Vapors are heavier than air. Used to manufacture other chemicals and as a fungicide.
공기와 물의 반응
Insoluble in water.
반응 프로필
Biphenyl is incompatible with oxidizers.
건강위험
Exposures to 1,1’-biphenyl cause acute effects with symptoms that include, but are not
limited to, polyuria, accelerated breathing, lacrimation, anorexia, weight loss, muscular
weakness, coma, fatty liver cell degeneration, and severe nephrotic lesions. Exposure to
biphenyl fumes for short periods of time causes nausea, vomiting, irritation of the eyes
and respiratory tract, and bronchitis. Breathing small amounts of 1,1’-biphenyl over long
periods of time causes damage to the liver and the nervous system of exposed workers.
Breathing the mists, vapors, or fumes may irritate the nose, throat, and lungs. Depending
on the concentration and duration of exposure, the symptoms include, but are not limited
to, sore throat, coughing, labored breathing, sneezing, a burning sensation, and the effects
of CNS depression. Symptoms may include headache, excitation, euphoria, dizziness,
incoordination, drowsiness, light-headedness, blurred vision, fatigue, tremors, convulsions, loss of consciousness, coma, respiratory arrest, and death, depending on the concentration and duration of exposure
화재위험
Combustible. Emits toxic fumes under fire conditions.
Safety Profile
Poison by intravenous route.Moderately toxic by ingestion. A powerful irritant byinhalation in humans. Human systemic effects byinhalation of very small amounts: flaccid paralysis, nauseaor vomiting, and other unspecified gastrointestinal effects.Que
잠재적 노출
Biphenyl is a fungicide (pesticide). It
is also used as a heat transfer agent, dye carrier, and as an
intermediate in organic synthesis.
신진 대사 경로
Biphenyl is a stable compound. It has a restricted use as a fungistat
applied to citrus packing material. This has generated studies on its
toxicity and metabolism in mammals and residue studies in stored fruit.
Only limited information on its environmental fate has been published.
저장
1,1’-Biphenyl should be kept stored in tightly closed containers in a cool, dry, isolated, and
properly ventilated area, away from heat, sources of ignition, incompatibles, and contact
with strong oxidizers.
운송 방법
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1—Poisonous materials, Technical
Name Required.
Purification Methods
Crystallise biphenyl from EtOH, MeOH, aqueous MeOH, pet ether (b 40-60o) or glacial acetic acid. Free it from polar impurities by passage through an alumina column in *benzene, followed by evaporation. The residue has been purified by distillation in a vacuum and by zone refining. Treatment with maleic anhydride removes anthracene-like impurities. It has been recrystallised from EtOH followed by repeated vacuum sublimation and passage through a zone refiner. [Taliani & Bree J Phys Chem 88 2351 1984, Beilstein 5 H 576, 5 I 271, 5 II 479, 5 III 1726, 5 IV 1807.]
비 호환성
Mist may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides.
폐기물 처리
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal, state,
and local environmental regulations must be observed.
주의 사항
Occupational workers should be careful during use and chemical management because the fi nely dispersed particles of 1,1’-biphenyl form explosive mixtures in air
비페닐 준비 용품 및 원자재
원자재
준비 용품
4-BIPHENYLSULFONYL CHLORIDE
polyquinoxaline
2-페닐페놀
4-벤조일바이페닐
4,4'-Diacetylbiphenyl
Raloxifene
4,4'-비스(브로모메틸)비페닐
6-bromoquinolin-4(3H)-one
β-에스트라디올
α-페닐-2-p-톨릴에틸아민
Fenbufen
디페닐-4-카본산
나프탈렌
4-브로모바이페닐
3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
4-(클로로페닐메틸)-1,1'-비페닐
6-BROMO-4-CHLOROQUINOLINE
2-Methyl-3-biphenylmethanol
브로마디올론
4,4'-비스(클로로메틸)-1,1'-바이페닐
3-(4'-Bromo[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro-1-naphthalenol
4-아미노디페닐
4-벤질바이페닐
6-클로로-2-(클로로에틸)-3-옥시도-4-페닐-퀴나졸린
brotianide
2-(2-AMINO-4-BIPHENYL)프로피오니트릴
바이포나졸
p-페닐페놀
fluorescent whitening agent ATS-X
비테르탄올
p-터페닐
2-페닐린돌
4-Acetylbiphenyl