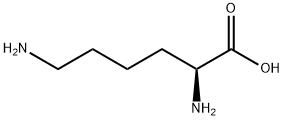
L-리신
|
|
L-리신 속성
- 녹는점
- 215 °C (dec.)(lit.)
- 알파
- D20 +14.6° (c = 6.5); D23 +25.9° (c = 2 in 6.0N HCl)
- 끓는 점
- 265.81°C (rough estimate)
- 밀도
- 1.1360 (rough estimate)
- FEMA
- 3847 | L-LYSINE
- 굴절률
- 26 ° (C=2, 5mol/L HCl)
- 저장 조건
- Keep in dark place,Inert atmosphere,Room temperature
- 용해도
- H2O: 0.1 g/mL, 투명, 무색
- 물리적 상태
- 분말 또는 결정
- 산도 계수 (pKa)
- 2.16(at 25℃)
- 색상
- 흰색에서 밝은 노란색
- 냄새
- 냄새 없는
- optical activity
- [α]20/D +26.0±1.0°, c = 2% in 6 M HCl
- 수용성
- 물에 용해됩니다. 에탄올, 에틸 에테르, 아세톤, 벤젠 및 일반적인 중성 용매에 용해되지 않습니다.
- Merck
- 14,5636
- JECFA Number
- 1439
- BRN
- 1722531
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- InChIKey
- KDXKERNSBIXSRK-YFKPBYRVSA-N
- LogP
- -3.05
- CAS 데이터베이스
- 56-87-1(CAS DataBase Reference)
- NIST
- Lysine(56-87-1)
- EPA
- Lysine (56-87-1)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 안전지침서 | 24/25 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | OL5540000 | ||
| TSCA | Yes | ||
| HS 번호 | 29224110 | ||
| 유해 물질 데이터 | 56-87-1(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-22657 |
| 그림문자(GHS): |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||
| 예방조치문구: |
|
L-리신 C화학적 특성, 용도, 생산
순도시험
(1) 용상 : 이 품목 1.0g을 물 40mL에 녹일 때, 그 액은 무색으로서 탁도는 거의 징명 이하이어야 한다.
(2) 비선광도 : 이 품목 약 2g을 정밀히 달아 6N 염산에 녹여 100mL로 하여 이 액의 선광도를 측정하고 다시 무수물로 환산할 때, 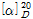 =+23.3~+29.3°이어야 한다.
=+23.3~+29.3°이어야 한다.
(3) 염화물 : 이 품목 0.07g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.2mL에 대응하는 양 이하이어야 한다.
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
확인시험
(1) 이 품목의 수용액(1→1,000) 5mL에 닌히드린용액(1→50) 1mL를 가하고 수욕상에서 3분간 가열하면 적자색을 나타낸다.
(2) 이 품목의 수용액은 알칼리성이다.
정량법
이 품목 약 0.2g을 정밀히 달아 개미산 3mL에 녹이고 빙초산(비수적정용) 50mL를 가하고 0.1N 과염소산용액으로 적정한다(지시약 : 크리스탈바이올렛․빙초산시액 1mL). 종말점은 액의 자색이 청색을 지나 녹색으로 변하는 점이다. 따로, 같은 방법으로 공시험을 한다.
0.1N 과염소산용액 1mL = 7.310mg C6H14N2O2
강열잔류물
이 품목의 강열잔류물시험법에 따라 시험할 때 0.2% 이하이어야 한다.
개요
See ι-LYSINE MONOCHLORIDE.화학적 성질
L-Lysine is an essential amino acid (a protein building block) that cannot be produced by the body from other nutri ents. It helps ensure adequate absorption of calcium and the formation of collagen for bone, cartilage and connective tissue. This compound is odorless.출처
Some natural food sources for l-lysine include lima beans, kidney beans, potatoes, corn, red meat, fish and milk.용도
L-Lysine is an essential amino acid used in human nutrition. It plays a vital role in calcium absorption, building muscle protein and recovering from surgery or sports injuries. It is used for the treatment of herpes infections and cold sores. Its derivatives lysine acetylsalicylate is utilized to treat pain as well as to detoxify the body after heroin use. It is an important additive to animal feed. Further, it is used in many foods, especially red meats, fish and dairy products.제조 방법
Produced by fermentation. Also produced by use of continuous ion exchange technology.정의
ChEBI: An L-alpha-amino acid; the L-isomer of lysine.일반 설명
L-Lysine is a basic amino acid.Safety Profile
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.Purification Methods
Crystallise L-lysine from aqueous EtOH. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2097-2122 1961, Kearley & Ingersoll J Am Chem Soc 73 5783 1951, Beilstein 4 IV 2717.]L-리신 준비 용품 및 원자재
원자재
발연황산
피크린산
피메리산
Soy bean Isoflavone Isoflavone 10-40%
요소
염산
수산화나트륨
감자전분
카세인
카프로락탐
Biochemistry
Peptide synthesis
활성화 챠콜
수산화칼슘
암모니아(가스)
Lysine, N2-[(phenylmethoxy)carbonyl]-
준비 용품
L-리신 공급 업체
글로벌( 816)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| DONBOO AMINO ACID COMPANY | +8613063595538 |
donboo@donboo.com | China | 9365 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 |
info@royal-chem.com | China | 114 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 |
admin01@hsnm.com.cn | China | 2118 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 |
fanfan@kangcang.com.cn | China | 340 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 |
admin@hbsaisier.cn | China | 747 | 58 |
L-리신 관련 검색:
L-(-)-트레오닌 L-(+)-아이소루신 L-(-)-페닐알라닌 핵산산 L-리신
Poly-L-lysine
L-Lysine dihydrochloride
L-Lysine hydrochloride
H-LYS-NH2 2HCL
L-Lysine S-(carboxymethyl)-L-cysteine
Ethyl 2,6-diaminohexanoate dihydrochloride
Methyl L-lysinate dihydrochloride
L-Carnosine
L-Malic acid
L-Tyrosine
L-Aspartic acid
Methyl (S)-(-)-lactate
L-Valine