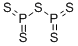삼황화사인
|
|
삼황화사인 속성
- 녹는점
- 286 °C
- 끓는 점
- 514°C
- 밀도
- 2.09 g/mL at 25 °C(lit.)
- 저장 조건
- Flammables area
- 용해도
- reacts with H2O; soluble in CS2
- 물리적 상태
- 가루
- 색상
- 노란색에서 녹색으로
- 수소이온지수(pH)
- 1 (10g/l, H2O, 20℃)
- 냄새
- 썩은 계란 냄새
- 수용성
- 물과 반응
- 안정성
- 흡습성, 수분 민감성
- InChIKey
- HWVAJUNEPOKXLF-UHFFFAOYSA-N
- CAS 데이터베이스
- 1314-80-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-20/22-29-50 | ||
| 안전지침서 | 61 | ||
| 유엔번호(UN No.) | UN 1340 4.3/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TH4375000 | ||
| F 고인화성물질 | 13-21 | ||
| 자연 발화 온도 | 142 °C | ||
| 위험 등급 | 4.3 | ||
| 포장분류 | II | ||
| HS 번호 | 28139000 | ||
| 유해 물질 데이터 | 1314-80-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 389 mg/kg LD50 dermal Rabbit 3160 mg/kg | ||
| IDLA | 250 mg/m3 | ||
| 기존화학 물질 | KE-12128 | ||
| 유해화학물질 필터링 | 97-1-214 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 오황화 인 및 이를 1% 이상 함유한 혼합물 |
삼황화사인 C화학적 특성, 용도, 생산
개요
Phosphorus pentasulfide, is a nonmetallic inorganic compound . It is a yellow to greenish-yellow crystalline mass with an odor similar to hydrogen sulfide. It is a dangerous fire risk and ignites by friction or in contact with water. Boiling point is 995°F (535°C) and ignition temperature is 287°F (141°C). It decomposes upon contact with water or moist air, liberating toxic and flammable hydrogen-sulfide gas. Specific gravity is 2.09, so it is heavier than water. It is toxic by inhalation, with a TLV of 1 mg/m3 of air. The four-digit UN identification number is 1340. The NFPA 704 designation is health 2, flammability 1, and reactivity 2. Primary uses are in insecticides, safety matches, ignition compounds, and sulfonation.화학적 성질
Phosphorus pentasulfide is a greenish-gray to yellow, crystalline solid with an odor of rotten eggs. The Odor Threshold is 0.005 ppm.
용도
Phosphorus pentasulfide is used in the manufacture of lubricant additives, pesticides, safety matches, and flotation agents.일반 설명
A greenish yellow solid with an odor of rotten eggs that may paralyze the sense of smell at hazardous concentrations in air. Density 2.04 g / cm3. Phosphorus pentasulfide is used for making lube oil additives, insecticides, flotation agents, safety matches, blown asphalt, and other products and chemicals.공기와 물의 반응
Highly flammable. May heat and spontaneously ignite in presence of moisture [Haz. Chem. Data. 1969]. Reaction with water forms toxic hydrogen sulfide gas and phosphoric acid. Dangerous when wet.반응 프로필
PHOSPHORUS PENTASULFIDE reacts vigorously with strong oxidants. Exothermically violent decomposition reaction with water, steam or acids to produce irritating fumes of phosphorus pentaoxide and highly toxic hydrogen sulfide gas. May ignite in contact with limited amounts of water [Haz. Chem. Data, 1975, p. 239]. Can be ignited by sparks or friction and its dust presents an explosion hazard in air at sufficient concentrations. Hydrogen sulfide gas evolved from reactions with water may also form explosive mixtures with air.건강위험
Phosphorus pentasulfide is an irritant to the skin and eyes. Inhalation of its vapors can cause irritation of the respiratory passage. Oral toxicity of this compound in rats was found to be moderate.LD50 value, oral (rats): 389 mg/kg (NIOSH 1986)
Phosphorus pentasulfide can readily produce highly toxic hydrogen sulfide in the presence of moisture. Other toxic products from its combustion are sulfur dioxide and phosphorus pentoxide (corrosive)..
화재위험
Special Hazards of Combustion Products: Products of combustion include sulfur dioxide and phosphorus pentoxide, which are irritating, toxic and corrosive.Safety Profile
A poison by ingestion. A severe eye and skin irritant. Readily liberates toxic hydrogen sulfide and phosphorus pentoxide and evolves heat on contact with moisture. Dangerous fire hazard in the form of dust when exposed to heat or flame. Spontaneous heating in the presence of moisture. Moderate explosion hazard in solid form by spontaneous chemical reaction. Reacts with water, steam, or acids to produce toxic and flammable vapors; can react vigorously with oxidizing materials. Incompatible with air, alcohols, water. To fight fire, use CO2snow, dry chemical, or sand. Used as an intermedate in manufacturing lubricant addltives, insecticides, and fertilizer agents. When heated to decomposition it emits highly toxic fumes of SO, and PO,. See also HYDROGEN SULFIDE.잠재적 노출
Phosphorus pentasulfide is used as an intermediate in the manufacture of lubricant additives; insecticides, flotation agents; lubricating oil; ignition compounds; and matches. It is also used to introduce sulfur into rubber, and organic chemicals, such as pharmaceuticals.운송 방법
UN1340 Phosphorus pentasulfate, free from yellow or white phosphorus, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material, 4.1-flammable solid.Purification Methods
Purify P2S5 by extraction and crystallisation with CS2, using a Soxhlet extractor, and is heated in a CO2 atmosphere at 150o to remove solvent. It liberates H2S in moist air. HARMFUL VAPOURS. [Klements in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 568 1963.]비 호환성
Flammable solid; dust may form explosive mixture with air. Contact with water forms phosphorus pentoxide and an explosive mixture of hydrogen sulfide with air. Phosphorus pentasulfide is incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, ammonia, strong acids, strong bases, alcohols. Reaction with water or moisture in the air releases heat, hydrogen sulfide (H2S), sulfur dioxide, and phosphoric acid. Pyrophoric hazard, may self-ignite in moist air.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Decompose with water, forming phosphoric acid, sulfuric acid and hydrogen sulfide. Provisions must be made for scrubbing hydrogen sulfide emissions. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring, then flush to sewer with large volumes of water.삼황화사인 준비 용품 및 원자재
원자재
준비 용품
디메틸티오린산클로라이드
BENZP-DINITRIDE-THIO-KETONE
4-SULFANYLNICOTINIC ACID
DICAPTHON
Dimethylphenyl dithiophosphate,sodium salt
6-머캡토퓨린
(2-(TRIFLUOROMETHYL)THIAZOL-5-YL)METHANOL
TOLRESTAT
카보페노티온
2,4-디메틸티아졸
포살론
술포텝
파라싸이온(파라티온)
4,5-DIAMINO-6-MERCAPTOPYRIMIDINE
3-METHYL-2-BENZOTHIAZOLINONE HYDRAZONE HYDROCHLORIDE
티오초산
설프로포스
6-(METHYLTHIO)PYRIMIDINE-4,5-DIAMINE
1,7-HEPTANEDIOL
2,5-디메틸티오펜
QUAZEPAM
포스멧
4-사이아노페놀
메틸티아졸린(2-)
O,O-dialkyl thiophosphoryl chloride
6-메르캅토퓨린
CYANOPHOS
싸이오아세트아마이드
Malathion emulsifiable concentrate
Lawesson 시약
O,O-디에틸 수소 포스포로디씨오에이트
클로르피리포스-메틸
6-METHOXY-2-METHYLBENZOTHIAZOLE
프롤(5-)-2니트로벤조티아졸
Antioxidant and antiseptic agent T202
디알킬디티오인산
프로티오포스
Furfuryl mercaptan
BROTIZOLAM
7-TRIFLUOROMETHYL-4-QUINOLINETHIOL
삼황화사인 공급 업체
글로벌( 209)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10326 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 |
ada@ipurechemical.com | CHINA | 3465 | 58 |
| Shanxi Xuanran Import and Export Trade Co., Ltd. | +8617735180244 |
mike_yan@xuanranglobal.com | CHINA | 4022 | 58 |