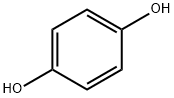
하이드로퀴논
|
|
하이드로퀴논 속성
- 녹는점
- 172-175 °C(lit.)
- 끓는 점
- 285 °C(lit.)
- 밀도
- 1.32
- 증기 밀도
- 3.81 (vs air)
- 증기압
- 1 mm Hg ( 132 °C)
- 굴절률
- 1.6320
- 인화점
- 165 °C
- 저장 조건
- Store below +30°C.
- 용해도
- H2O: 50 mg/mL, 투명
- 물리적 상태
- 바늘 모양의 결정 또는 결정성 분말
- 산도 계수 (pKa)
- 10.35(at 20℃)
- 색상
- 흰색에서 황백색까지
- 냄새
- 냄새 없는
- 수용성
- 70g/L(20℃)
- 감도
- Air & Light Sensitive
- Merck
- 14,4808
- BRN
- 605970
- Henry's Law Constant
- (x 10-9 atm?m3/mol): <2.07 at 20 °C (approximate - calculated from water solubility and vapor pressure)
- 노출 한도
- NIOSH REL: 15-min ceiling 2, IDLH 50; OSHA PEL: TWA 2; ACGIH TLV: TWA 2 (adopted).
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 강염기, 산소, 철염과 호환되지 않습니다. 가볍고 공기에 민감합니다. 공기 중에서 변색됨.
- InChIKey
- QIGBRXMKCJKVMJ-UHFFFAOYSA-N
- LogP
- 0.59 at 20℃
- CAS 데이터베이스
- 123-31-9(CAS DataBase Reference)
- IARC
- 3 (Vol. 15, Sup 7, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-40-41-43-50-68-R68-R50-R43-R41-R40-R22 | ||
| 안전지침서 | 26-36/37/39-61-S61-S36/37/39-S26 | ||
| 유엔번호(UN No.) | 2662 | ||
| OEL | Ceiling: 2 mg/m3 [15-minute] | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | MX3500000 | ||
| 자연 발화 온도 | 930 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 29072210 | ||
| 유해 물질 데이터 | 123-31-9(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 320 mg/kg (Woodard) | ||
| IDLA | 50 mg/m3 | ||
| 기존화학 물질 | KE-35112 | ||
| 유해화학물질 필터링 | 2010-1-610 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 하이드로퀴논 및 이를 2.5% 이상 함유한 혼합물 |
하이드로퀴논 C화학적 특성, 용도, 생산
개요
하이드로 퀴논은 유기 화합물입니다. 하이드로 퀴논은 하이드 록실 형태로 치환 된 수소의 벤젠 화합물의 두 대위법입니다. 흰색 결정체. 하이드로 퀴논 (hydroquinone) 독성 물질 1g과 성인 1g은 두통, 현기증, 이명, 창백한 얼굴과 같은 증상을 나타낼 수 있습니다.용도
하이드로 퀴논은 주로 흑백 콘트라스트, 안트라 퀴논 염료, 아조 염료, 고무 보호제, 안정제 및 산화 방지제 제조에 사용됩니다.개요
Hydroquinone (HQ) is produced by the oxidation of aniline or phenol, by the reduction of quinone, or from a reaction of acetylene and carbon monoxide. Hydroquinone occurs naturally as a glucose ether, also known as arbutin, in the leaves of many plants and in fruits, as well as one of the agents used in the defense mechanism of the bombardier beetle, family Carabidae.화학적 성질
Hydroquinone, a colorless, hexagonal prism, has been reported to be a good antimitotic and tumor-inhibiting agent. It is a reducing agent used in a photographic developer, which polymerizes in the presence of oxidizing agents. In the manufacturing industry it may occur include bacteriostatic agent, drug, fur processing, motor fuel, paint, organic chemicals, plastics, stone coating, and styrene monomers.물리적 성질
Colorless to pale brown, odorless, hexagonal crystals용도
hydroquinone is a pigment-lightening agent used in bleaching creams. Hydroquinone combines with oxygen very rapidly and becomes brown when exposed to air. Although it occurs naturally, the synthetic version is the one commonly used in cosmetics. Application to the skin may cause allergic reaction and increase skin sun sensitivity. Hydroquinone is potentially carcinogenic and is associated with causing ochronosis, a discoloration of the skin. The u.S. FDA has banned hydroquinone from oTC cosmetic formulations, but allows 4 percent in prescription products. Its use in cosmetics is prohibited in some european countries and in Australia.정의
ChEBI: A benzenediol comprising benzene core carrying two hydroxy substituents para to each other.Indications
Hydroquinone interferes with the production of the pigment melanin by epidermal melanocytes through at least two mechanisms: it competitively inhibits tyrosinase, one of the principal enzymes responsible for converting tyrosine to melanin, and it selectively damages melanocytes and melanosomes (the organelles within which melanin is stored).생산 방법
There are three current manufacturing processes for HQ: oxidative cleavage of diisopropylbenzene, oxidation of aniline, and hydroxylation of phenol.Diisopropylbenzene is air oxidized to the intermediate diisopropylbenzene bishydroperoxide. This hydroperoxide is purified by extraction and reacted further to form hydroquinone. The purified product is isolated by filtration and packaged. The process can be almost entirely closed, continuous, computer-controlled, and monitored.
HQcan also be prepared by oxidizing aniline to quinone in the presence of manganese dioxide and sulfuric acid. p-Benzoquinone is then reduced to HQ using iron oxide. The resulting hydroquinone is crystallized and dried. The process occurs in a closed system.
HQis also manufactured by hydroxylation of phenol using hydrogen peroxide as a hydroxylation agent. The reaction is catalyzed by strong mineral acids or ferrous or cobalt salts.
World Health Organization (WHO)
Hydroquinone was introduced in 1965 as a topical depigmenting agent for hyperpigmentation. At high concentrations hydroquinone is corrosive and in most countries has been restricted to the level of approximately 2% and limited to the period of less than 2 months. Additional consideration for restrictive action is that animal experiments have also demonstrated carcinogenic and mutagenic potential of hydroquinone.일반 설명
Light colored crystals or solutions. May irritate the skin, eyes and mucous membranes. Mildly toxic by ingestion or skin absorption.공기와 물의 반응
Darkens on exposure to air and light. Miscible in water. Solutions become brown in air due to oxidation. Oxidation is very rapid in the presence of alkali.반응 프로필
Hydroquinone is a slight explosion hazard when exposed to heat. Incompatible with strong oxidizing agents. Also incompatible with bases. Hydroquinone reacts with oxygen and sodium hydroxide. Reacts with ferric salts . Hot and/or concentrated NaOH can cause Hydroquinone to decompose exothermically at elevated temperature. (NFPA Pub. 491M, 1975, 385)위험도
Toxic by ingestion and inhalation, irritant. Questionable carcinogen.건강위험
Hydroquinone is very toxic; the probable oral lethal dose for humans is 50-500 mg/kg, or between 1 teaspoon and 1 ounce for a 150 lb. person. It is irritating but not corrosive. Fatal human doses have ranged from 5-12 grams, but 300-500 mg have been ingested daily for 3-5 months without ill effects. Death is apparently initiated by respiratory failure or anoxia.화재위험
Dust cloud may explode if ignited in an enclosed area. Hydroquinone can react with oxidizing materials and is rapidly oxidized in the presence of alkaline materials. Oxidizes in air.색상 색인 번호
Hydroquinone is used in photography developers (black and white, X-ray, and microfilms), in plastics, in hair dyes as an antioxidant and hair colorant. Hydroquinone is found in many skin bleaching creams.Clinical Use
Hydroquinone is applied topically to treat disorders characterized by excessive melanin in the epidermis, such as melasma. In the United States, nonprescription skin-lightening products contain hydroquinone at concentrations of 2% or less; higher concentrations are available by prescription.부작용
The incidence of adverse effects with hydroquinone increases in proportion to its concentration. A relatively common side effect is local irritation, which may actually exacerbate the discoloration of the skin being treated. Allergic contact dermatitis occurs less commonly. A rare but more serious complication is exogenous ochronosis, in which a yellow-brown pigment deposited in the dermis results in blue-black pigmentation of the skin that may be permanent.Carcinogenicity
No case reports of cancer associated with HQ exposure have been published.Purification Methods
Crystallise quinol from acetone, *benzene, EtOH, EtOH/*benzene, water or acetonitrile (25g in 30mL), preferably under nitrogen. Dry it under vacuum. [Wolfenden et al. J Am Chem Soc 109 463 1987, Beilstein 6 H 836, 6 IV 5712.]하이드로퀴논 준비 용품 및 원자재
원자재
준비 용품
포수클로랄
2-t-부틸하이드로퀴논
2,5-Dimethoxy-4-chloroaniline
에틸3,3-디메틸펜트-4-엔-1-오에이트
플루아지포프-P-뷰틸
2-하이드록시프로필아크릴레이트
1-(2,5-디메톡시페닐)-2-옥시미노-1-프로파논
7-HYDROXYISOFLAVONE
4-CHLORO-8-(TRIFLUOROMETHYL)QUINOLINE
부틸레이티드 하이드록시 아니솔
뷰틸 시아노아크릴산 염
2-아미노-1-(2,5-다이메톡시페닐)-1-프로파논
피레녹신
2- (디 사이클로 헥실 포스 피노) -2 ', 4', 6'- 트리 -i- 프로필 -1,1'- 비 페닐
3-METHYL-2,5-DIHYDROTHIOPHENE-1,1-DIOXIDE
C.I. 디스퍼스 오렌지 30
ammonium manganous sulfate
1,2,4-Trimethoxybenzene
Chloro(1,5-cyclooctadiene)iridium(I) dimer
이산화 2,5-다이하이드로싸이오펜
플루아지포프-뷰틸
2-하이드록시에틸 메트아크릴산
methyl(±)cis,trans-2,2-dimethyl-3-(2-methyl-1-propenyl cyclopropane carboxylate)
N-NONYL ACRYLATE
N-(4-아미노-2,5-다이에톡시페닐)벤즈아마이드
2,3-디메틸-1,3-부타디엔
2,5-다이하이드록시벤조익애씨드
4-[(6-Chloro-1,3-benzoxazol-2-yl)oxy]phenol
4-페녹시페놀
1,4-디메톡시벤젠
히드로퀴논 디아세테이트
2-CHLOROPYRIMIDINE-4-CARBONITRILE
2,5-디메톡시아닐린
2,5-디-t-부틸하이드로퀴논
에틸 크리산테메이트
Quizalofop-p-ethyl
1,2-NAPHTHALIC ANHYDRIDE
카프로산 알릴
(1,1-DIMETHYL-PROP-2-YNYL)-HYDRAZINE
4-에텐일페놀 아세트산
하이드로퀴논 공급 업체
글로벌( 916)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Changshu New Venture Imp.&Exp. Co.,Ltd. | 0512-52678575 13776233393 |
nvchem_deng@163.com | China | 212 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34571 | 58 |
| Wuhan Biet Co., Ltd. | +8617320528784 |
min@biet.com.cn | China | 41 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615350571055 |
Sibel@weibangbio.com | China | 5848 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5872 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 |
angelia@hbmojin.com | China | 1069 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |