삼산화황
|
|
삼산화황 속성
- 녹는점
- 16.8 °C(lit.)
- 끓는 점
- 44.8 °C
- 밀도
- 1.97 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.8 (vs air)
- 증기압
- 280 mm Hg ( 25 °C)
- 저장 조건
- Indoors
- 용해도
- reacts with H2O
- 물리적 상태
- 무색의 액체
- 색상
- 화이트 바늘 크리스탈
- 수용성
- 물 에 용해
- Merck
- 13,9069
- Dielectric constant
- 3.1(18℃)
- 안정성
- 안정적인. 유기 물질, 미세 분말 금속, 염기, 물, 시안화물 및 기타 다양한 화학 물질과 호환되지 않습니다. 물, 산소 및 기타 화학 물질과 격렬하게 반응합니다. 사용하기 전에 전체 데이터 시트를 읽으십시오.
- CAS 데이터베이스
- 7446-11-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,T+,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-8-14-26-34-37-35 | ||
| 안전지침서 | 53-17-26-36/37/39-45-8-25-30 | ||
| 유엔번호(UN No.) | UN 1829 8/PG 1 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | WT4830000 | ||
| 위험 등급 | 8 | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 7446-11-9(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-32690 |
삼산화황 C화학적 특성, 용도, 생산
화학적 성질
Sulfur trioxide, S03, also known as sulfuric anhydride, needles or polymer, exists in a number of modifications that differ in molecular species and crystalline form. It has a white,ice-like modification that melts at 16°C (61°F) and two other as bestos-like forms that melt at the higher temperatures of 33 and 62°C (90 and 144 °F). The colorless liquid or gas form has irritating, toxic fumes and boils at 45 °C (112 °F).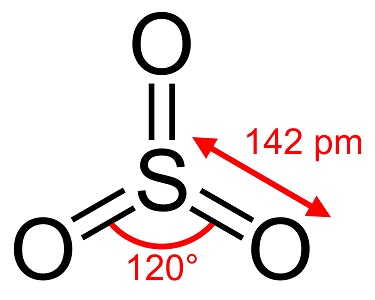
Sulfur trioxide is a highly reactive substance, a strong oxidizing agent,and a fire hazard. It reacts with metallic oxides to form sulfates and with water to form sulfuric acid. Sulfur trioxide is used for sulfonation.
물리적 성질
Colorless liquid at ambient temperature and atmospheric pressure; fumes in air.Sulfur trioxide tends to polymerize, particularly in the presence of traces of water or sulfuric acid. The rate of its polymerization, however, decreasesgreatly as its freezing point is approached. Solid (polymeric) sulfur trioxide exists in three polymorphic phases: alpha-, beta- and gamma- modifications.
The alpha phase is made up of ice-like needles having polymeric crosslinked structure. It melts at 62.3°C and has a vapor pressure of 73 torr at 25°C.
The beta phase is a metastable allotrope with white, asbestos-like, lustrous needles consisting of polymeric molecules, melting at 32.5°C, and with vapor pressure 344 torr at 25°C.
The gamma modification at ordinary temperatures can exist in solid or liquid form. In solid form it is a colloidal ice-like mass melting at 16.8°C. In the liquid form it has a density of 1.9224 g/mL, boiling at 44.8°C. It has a vapor pressure of 433 torr at 25°C. The gamma phase consists of both cyclic trimer and monomer molecules. When solid sulfur trioxide melts, it converts to its gamma phase which on solidification changes to alpha modification.
Critical temperature of SO3 is 217.8°C; critical pressure 80.97 atm; critical density 0.63 g/cm3; the dielectric constant of liquid SO3 at 18°C is 3.11.
Sulfur trioxide dissolves in water forming sulfuric acid and generating large heat.
용도
Sulfur trioxide is used as an intermediate in the manufacture of sulphuric acid and oleum for sulphonation, in particular, of dyes and dye-stuffs, and for the production of anhydrous nitric acid and explosives. Solid Sulfur trioxide is marketed under such names as Sulphan and Triosul, and is used primarily for sulphonation of organic acids. Sulphur tetrafluoride is a fluorinating agent. Sulphur hexafluoride serves as a gaseous insulator in high-voltage electric installations. Sulphyryl fluoride is used as an insecticide and a fumigant.Sulfonation of organic compounds, especially nonionic detergents, solar energy collectors. It is usually generated in the plant where it is to be used.
생산 방법
Sulfur trioxide is produced as an intermediate in manufacturing sulfuric acid by the contact process (See Sulfuric Acid). The process involves catalytic oxidation of sulfur dioxide to trioxide.Sulfur trioxide is prepared in the laboratory by heating fuming sulfuric acid, condensing its vapors, and collecting in a cool receiver. When vapors are condensed below 27°C in the presence of trace moisture, all three polymorphic phases of SO3 are produced. They can be separated by fractional distillation. Condensation of the vapors above 27°C forms the liquid variety of gamma-sulfur trioxide.
일반 설명
Sulfur trioxide, is a colorless to white crystalline solid which will fume in air. Often shipped with inhibitor to prevent polymerization. Sulfur trioxide reacts violently with water to form sulfuric acid with the release of heat. Sulfur trioxide is corrosive to metals and tissue. Sulfur trioxide causes eye and skin burns. Ingestion causes severe burns of mouth esophagus and stomach. The vapor is very toxic by inhalation. Sulfur trioxide is a fire risk when in contact with organic materials such as wood, cotton, fiberboard, etc.공기와 물의 반응
Combines with water with explosive force, forming sulfuric acid due to its acidity Sulfur trioxide chars most organic substances. On exposure to air Sulfur trioxide absorbs moisture rapidly, emitting dense white fumes [Merck 11th ed. 1989].반응 프로필
The reaction of Sulfur trioxide and oxygen difluoride is very vigorous and explosions occur if the reaction is carried out in the absence of a solvent [J. Chem. Eng. Data 13(4):529-531. 1968]. The reaction of Sulfur trioxide in excess with tetrafluoroethylene causes explosive decomposition to carbonyl fluoride and sulfur dioxide [Chem. Eng. News 49(22):3. 1971]. The reaction of anhydrous perchloric acid with Sulfur trioxide is violent and accompanied by the evolution of considerable heat (Pascal 16:300 1931-34). Liquid Sulfur trioxide reacts violently with nitryl chloride, even at 75° C. The reaction of Sulfur trioxide and lead oxide causes white luminescence [Mellor 7:654 1946-47]. The combination of iodine, pyridine, Sulfur trioxide, and formamide developed a gas over pressurization after several months. This is due to the slow formation of sulfuric acid, from external water or dehydration of the formamide to hydrogen cyanide.위험도
Oxidizing agent, fire risk in contact with organic materials, an explosive increase in vapor pressure occurs when the α form melts. The anhydride combines with water, forming sulfuric acid and evolving heat. Highly toxic, strong irritant to tissue.건강위험
Sulfur trioxide is highly toxic. It is an irritant and corrosive to mucous membranes. Poisonous if inhaled or swallowed. Contact causes severe burns to skin and eyes.화재위험
Fire risk in contact with organic materials. An explosive increase in vapor pressure occurs when the alpha form melts. Combines with water with explosive violence, forming sulfuric acid. May ignite other combustible materials (wood, paper, oil, etc.). Flammable poisonous gases may accumulate in tanks and hopper cars. Runoff to sewer may create fire or explosion hazard. Forms sulfuric acid on contact with water. Avoid water and organic materials. On exposure to air, Sulfur trioxide absorbs moisture and emits dense white fumes.Safety Profile
Poison by inhalation. Human systemic effects by inhalation: cough and other pulmonary and olfactory changes. A corrosive irritant to skin, eyes, and mucous membranes. Violent reaction with O2F2, PbO, NClO2, HClO4, P, tetrafluorethylene, acetonitrile, sulfuric acid, dimethyl sulfoxide, dioxan, water, diphenylmercury, formamide, iodine, pyridine, metal oxides. Reacts with steam to form corrosive, toxic fumes of sulfuric acid. When heated to decomposition it emits toxic fumes of SO,. See also SULFURIC ACID.잠재적 노출
Sulfur trioxide is used as a sulfating and sulfonating agent for detergent, lubricating oil additives, and other organic compounds; in solar energy collectors. It is also used as an intermediate in sulfuric acid manufacture and in making explosives.저장
The vapour pressure of Sulfur trioxide rises rapidly with increasing temperatures and, when the α-form melts, the pressure rise is explosive; consequently transport and storage containers must withstand pressures of 10 to 15 atm. Sulfur trioxide reacts vigorously and highly exothermically with water to produce hydrosulphuric acid. When exposed to moist air, it fumes and forms a mist of sulphuric acid which eventually fills all the available space; it also corrodes metals. It is a powerful oxidizing agent and, in the liquid phase, carbonizes organic materials.운송 방법
UN1829 Sulfur trioxide, stabilized, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous Inhalation Hazard, Inhalation Hazard Zone B.비 호환성
Combustible and Corrosive. A strong oxidizer. Reacts violently with water, steam or moisture, releasing corrosive hydrosulfuric acid. Violent reactions occur on contact with strong bases; strong acids, chemically active metals; reducing agents; finely divided metal; cyanides, nitrates, picrates, fulminates, chlorates, sulfides, carbides, phosphorus, dioxygen difluoride, barium oxide; lead oxide; diphenyl mercury; alcohols, nitryl chloride; acetonitrile, dioxane, tetrafluoroethylene.폐기물 처리
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.삼산화황 준비 용품 및 원자재
원자재
준비 용품
5-[2-(METHYLTHIO)PYRIMIDIN-4-YL]THIOPHENE-2-SULFONYL CHLORIDE
고급 알코올 황산에스테르염
Industrial sulfuric acid
2-(4-모르폴리노)에탄설폰산
stearyltoluene sodium sulfonate
sec-alkyl sodium sulfate
페놀
도데실벤젠설폰산 나트륨
4-(2-Methoxyethyl)Phenol
레조시놀
monoethanolamine dodecyl sulfate
클로로술폰산
3-OXO-3-THIOPHEN-2-YL-PROPIONIC ACID ETHYL ESTER
설파믹산
메타닐산
alkyl phenylsufonate
도데실벤젠술폰산칼슘
염화구리(Ⅰ)
p-페닐페놀
산화크로뮴
소듐사이클라메이트
염화 티오닐
황산
디메틸설페이트
삼산화황 공급 업체
글로벌( 60)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| Richest Group Ltd | 18017061086 |
oled@richest-group.com | CHINA | 5601 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9320 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 |
dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23931 | 58 |