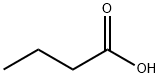
Butyric Acid
- CAS No.
- 107-92-6
- Chemical Name:
- Butyric Acid
- Synonyms
- BUTANOIC ACID;butyrate;C4;N-BUTYRIC ACID;N-BUTYRATE;N-BUTANOIC ACID;C4A;FEMA 2221;n-Butyric;Buttersαure
- CBNumber:
- CB3459186
- Molecular Formula:
- C4H8O2
Lewis structure
- Molecular Weight:
- 88.11
- MDL Number:
- MFCD00002814
- MOL File:
- 107-92-6.mol
- MSDS File:
- SDS
| Melting point | ?6-?3 °C (lit.) |
|---|---|
| Boiling point | 162 °C (lit.) |
| Density | 0.964 g/mL at 25 °C (lit.) |
| vapor density | 3.04 (vs air) |
| vapor pressure | 0.43 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2221 | BUTYRIC ACID |
| Flash point | 170 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Soluble), Isopropanol (Sparingly), Methanol (Slightly);Miscible with water, Propylene glycol, Glycerin, alcohol and oils. |
| pka | 4.83(at 25℃) |
| form | Liquid |
| Specific Gravity | 0.960 (20/4℃) |
| color | Clear colorless |
| Odor | at 1.00 % in dipropylene glycol. sharp acetic cheese butter fruit |
| PH | 3.94(1 mM solution);3.42(10 mM solution);2.92(100 mM solution); |
| Odor Type | cheesy |
| Odor Threshold | 0.00019ppm |
| explosive limit | 2-12.3%(V) |
| Water Solubility | MISCIBLE |
| Merck | 14,1593 |
| JECFA Number | 87 |
| BRN | 906770 |
| Dielectric constant | 3.0(Ambient) |
| Stability | Flammable. Incompatible with strong oxidizing agents, aluminium and most other common metals, alkalies, reducing agents. |
| InChIKey | FERIUCNNQQJTOY-UHFFFAOYSA-N |
| LogP | 1.1 at 25℃ |
| Substances Added to Food (formerly EAFUS) | BUTYRIC ACID |
| FDA 21 CFR | 182.63 |
| CAS DataBase Reference | 107-92-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 40UIR9Q29H |
| NIST Chemistry Reference | Butanoic acid(107-92-6) |
| EPA Substance Registry System | Butyric acid (107-92-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314 | |||||||||
| Precautionary statements | P270-P280-P301+P312-P301+P330+P331-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36-45 | |||||||||
| RIDADR | UN 2820 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | ES5425000 | |||||||||
| F | 13 | |||||||||
| Autoignition Temperature | 824 °F | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 60 19 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in rats: 8.79 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Butyric Acid price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W222119 | Butyric Acid natural, ≥99%, FCC, FG | 107-92-6 | 1kg | $118 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222119 | Butyric Acid natural, ≥99%, FCC, FG | 107-92-6 | 5kg | $300 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222119 | Butyric Acid natural, ≥99%, FCC, FG | 107-92-6 | 10Kg | $385 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222119 | Butyric Acid natural, ≥99%, FCC, FG | 107-92-6 | 25kg | $644 | 2024-03-01 | Buy |
| Sigma-Aldrich | W222100 | Butyric Acid ≥99%, FG | 107-92-6 | 1kg | $108 | 2024-03-01 | Buy |
Butyric Acid Chemical Properties,Uses,Production
Description
Butyric acid is a carboxylic acid also classified as a fatty acid. It exists in two isomeric forms as shown previously, but this entry focuses on n-butyric acid or butanoic acid. It is a colorless, viscous, rancid-smelling liquid that is present as esters in animal fats and plant oils. Butyric acid exists as a glyceride in butter, with a concentration of about 4%; dairy and egg products are a primary source of butyric acid. When butter or other food products go rancid, free butyric acid is liberated by hydrolysis, producing the rancid smell. It also occurs in animal fat and plant oils.
Chemical Properties
n-Butyric acid has a persistent, penetrating, rancid, butter-like odor and burning, acid taste.
Chemical Properties
Butyric acid is a fatty acid occurring in the form of esters in animal fats. The triglyceride of butyric acid makes up 3% to 4% of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis, leading to the unpleasant odor. It is an important member of the fatty acid subgroup called short- chain fatty acids. Butyric acid is a medium-strong acid that reacts with bases and strong oxidants, and attacks many metals.
The acid is an oily, colorless liquid that is easily soluble in water, ethanol, and ether, and can be separated from an aqueous phase by saturation with salts such as calcium chloride. It is oxidized to carbon dioxide and acetic acid using potassium dichromate and sulfuric acid, while alkaline potassium permanganate oxidizes it to carbon dioxide. The calcium salt, Ca(C4H7O2)2·H2O, is less soluble in hot water than in cold.
Butyric acid has a structural isomer called isobutyric acid (2-methylpropanoic acid).
Chemical Properties
Butyric acid (from Greek meaning "butter"), also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo's milk, butter, Parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 ppb, whereas humans can detect it in concentrations above 10 ppm.
Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it . The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.
Chemical Properties
Butyric acid is a combustible, oily liquid with an unpleasant odor. The Odor Threshold is 0.0001 ppm.
Chemical Properties
Butyric acid, C3H7COOH, a colorless liquid with an obnoxious odor, occurring in spoiled butter.It miscible with water, alcohol, and ether.It is used in the synthesis of butyrate ester perfume and flavor ingredients and in disinfectants and pharmaceuticals,
Occurrence
Normally occurs in butter as a glyceride. It has been reported found in the essential oils of citronella Ceylon, Eucalyptus globules, Araucaria cunninghamii, Lippia scaberrima, Monarda fistulosa, cajeput, Heracleum giganteum, lavender, Hedeoma pulegioides, valerian, nutmeg, hops, Pastinaca sativa, Spanish anise and others. It has been identified in strawberry aroma, apricot, American cranberry, sour cherry, black currants, butter, milk, strawberry jam, cheeses (blue, cheddar, feta, Swiss, Camembert and romano), raspberry, papaya, coffee mutton, beer, rum, bourbon whiskey and cider.
History
Butyric acid gets its name from the Latin butyrum, or butter. It was discovered by Adolf Lieben (1836–1914) and Antonio Rossi in 1869. Butyric acid is one of the simplest fatty acids.
Uses
Butyric acid is used in the preparation of various butyrate esters. Low-molecular-weight esters of butyric acid, such as methyl butyrate, have mostly pleasant aromas or tastes. As a consequence, they find use as food and perfume additives. It is also used as an animal feed supplement, due to the ability to reduce pathogenic bacterial colonization. It is an approved food flavoring in the EU FLAVIS database (number 08.005).
Due to its powerful odor, it has also been used as a fishing bait additive. Many of the commercially available flavors used in carp (Cyprinus carpio) baits use butyric acid as their ester base; however, it is not clear whether fish are attracted by the butyric acid itself or the substances added to it. Butyric acid was, however, one of the few organic acids shown to be palatable for both tench and bitterling. The substance has also been used as a stink bomb by Sea Shepherd Conservation Society to disrupt Japanese whaling crews, as well as by anti-abortion protesters to disrupt abortion clinics.
Uses
It is used in plastics as a raw material for the cellulose acetate butyrate (CAB). Other uses of butyric acid are in disinfectants, pharmaceuticals, and feed supplements for plant and animals. Butyric acid derivatives play an important role in plant and animal physiology.
Uses
Butyric Acid is a fatty acid that is commonly obtained from butter fat. it has an objectionable odor which limits its uses as a food acid- ulant or antimycotic. it is an important chemical reactant in the manufacture of synthetic flavoring, shortening, and other edible food additives. in butter fat, the liberation of butyric acid which occurs during hydrolytic rancidity makes the butter fat unusable. it is used in soy milk-type drinks and candies.
Definition
ChEBI: A straight-chain saturated fatty acid that is butane in which one of the terminal methyl groups has been oxidised to a carboxy group.
Definition
A colorless liquid carboxylic acid. Esters of butanoic acid are present in butter.
Preparation
Obtained by fermentation of starches and molasses with selective enzymes (Granulo saccharobutyricum); it is subsequently isolated as the calcium salt.
Production Methods
Butyric acid is produced by oxidation of butyraldehyde (CH3(CH2)2CHO) or butanol (C4H9OH). It can also be formed biologically by the oxidation of sugar and starches using bacteria.
Production Methods
Butyric Acid is industrially prepared by the fermentation of sugar or starch, brought about by the addition of putrefying cheese, with calcium carbonate added to neutralize the acids formed in the process. The butyric fermentation of starch is aided by the direct addition of Bacillus subtilis. Salts and esters of the acid are called butyrates or butanoates.
Butyric acid or fermentation butyric acid is also found as a hexyl ester hexyl butyrate in the oil of Heracleum giganteum (a type of hogweed) and as the octyl ester octyl butyrate in parsnip (Pastinaca sativa); it has also been noticed in skin flora and perspiration.
Aroma threshold values
Detection: 240 ppb to 4.8 ppm
Taste threshold values
Taste characteristics at 250 ppm: acidic, sour, cheesy, dairy, creamy with a fruity nuance.
General Description
A colorless liquid with a penetrating and unpleasant odor. Flash point 170°F. Corrosive to metals and tissue. Density 8.0 lb /gal.
Air & Water Reactions
Water soluble.
Reactivity Profile
(3R,4S)-1-Benzoyl-3-(1-methoxy-1-methylethoxy)-4-phenyl-2-azetidinone can react with oxidizing agents. Incandescent reactions occur with chromium trioxide above 212°F. Also incompatible with bases and reducing agents. May attack aluminum and other light metals .
Hazard
Strong irritant to skin and tissue.
Health Hazard
Inhalation causes irritation of mucous membrane and respiratory tract; may cause nausea and vomiting. Ingestion causes irritation of mouth and stomach. Contact with eyes may cause serious injury. Contact with skin may cause burns; chemical is readily absorbed through the skin and may cause damage by this route.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Biotechnological Applications
Butyrate is produced as end - product of a fermentation process solely performed by obligate anaerobic bacteria. Fermented Kombucha "tea" includes butyric acid as a result of the fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861.
The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is then oxidized into acetyl coenzyme A using a unique mechanism that involves an enzyme system called pyruvate - ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of elemental hydrogen (H2) are formed as waste products from the cell.
Safety Profile
Moderately toxic by ingestion, skin contact, subcutaneous, intraperitoneal, and intravenous routes. Human mutation data reported. Severe skin and eye irritant. A corrosive material. Combustible liquid. Could react with oxidizing materials. Incandescent reaction with chromium trioxide above 100'. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
The United States Environmental Protection Agency rates and regulates butyric acid as a toxic substance.
Personal protective equipment such as rubber or PVC gloves, protective eye goggles, and chemical-resistant clothing and shoes are used to minimize risks when handling butyric acid.
Inhalation of butyric acid may result in soreness of throat, coughing, a burning sensation and laboured breathing. Ingestion of the acid may result in abdominal pain, shock, and collapse. Physical exposure to the acid may result in pain, blistering and skin burns, while exposure to the eyes may result in pain, severe deep burns and loss of vision.
Potential Exposure
In manufacture of butyrate esters, some of which go into artificial flavoring. Incompatibilities: May form explosive mixture with air. Incompatible with sulfuric acid, caustics, ammonia, aliphatic amines; isocyanates, strong oxidizers; alkylene oxides; epichlorohydrin
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting.
Environmental Fate
The most probable mechanism of toxicity is the formation of an acid proteinate following exposure to high concentrations. Such complexes result in an inhibition of protein function and disruption of cellular homeostasis. Butyric acid induces apoptosis by production of ceramide and reactive oxygen species in the mitochondria followed by activation of JNK in mitogen activated protein (MAP) kinase cascades. Butyric acid has two contrasting functional roles. As a product of fermentation within the human colon, it serves as the most important energy source for normal colorectal epithelium. It also promotes differentiation of cultured malignant cells. A switch from aerobic to anaerobic metabolism accompanies neoplastic transformation in the colorectum. The separate functional roles for n-butyrate may reflect the different metabolic activities of normal and neoplastic tissues. Deficiency of n-butyrate, coupled with the increased energy requirements of neoplastic tissue, may promote the switch to anaerobic metabolism. n-Butyrate was previously found to increase epidermal growth factor receptor binding in primary cultures of rat hepatocytes. It was shown that butyrate and dexamethasone synergistically modulate the surface expression of epidermal growth factor receptors. The butyrate-induced enhancement of highaffinity epidermal growth factor bindingwas slight in the absence of glucocorticoid, but was strongly and dose-dependently amplified by dexamethasone. Butyrate counteracted the inhibition by insulin of the dexamethasone-induced increase in epidermal growth factor binding. The results indicate that the glucocorticoid has a permissive effect on a butyrate-sensitive process that determines the surface expression of the high-affinity class of epidermal growth factor receptors.
storage
Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Protectagainst physical damage. Outside or detached storage is preferred. Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Prior toworking with butyric acid you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from incompatiblematerials listed above. Metal containers involving the transfer of this chemical should be grounded and bonded. Drumsmust be equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof this chemical. Sources of ignition, such as smoking andopen flames, are prohibited where this chemical is used,handled, or stored in a manner that could create a potentialfire or explosion hazard.
Shipping
UN2820 Butyric acid, Hazard class: 8; Labels: 8—Corrosive material. UN2529 Isobutyric acid, Hazard Class: 3; Labels: 3—Flammable liquid, 8—Corrosive material
Purification Methods
Distil the acid, them mix it with KMnO4 (20g/L), and fractionally redistil, discarding the first third of the distillate [Vogel J Chem Soc 1814 1948]. [Beilstein 2 IV 779.]
Toxicity evaluation
Butyric acid is not environmentally persistent as it is
biodegradable in aqueous media, volatilizes from surface
waters at a moderate rate, and readily undergoes photodegradation
in the atmosphere. n-Butanoic acid may be
susceptible to biodegradation in terrestrial and aquatic environments
based on the observed degradation of 72% after 5 h
when incubated with activated sludge. At an initial concentration
of 100 mg l-1, n-butanoic acid displayed a 72% theoretical
biological oxygen demand (BODT) after 5 h when incubated
with activated sludge. n-Butanoic acid at an initial concentration
of 5 ppm displayed a BODT of 76.6% in freshwater and
72.4% in seawater after 5 days. n-Butanoic acid had a BODT of
17.4, 23.8, 26.2, and 27.7% after 6, 12, 18, and 24 h, respectively,
when incubated with an activated sludge seed at an
initial concentration of 500 ppm. In a screening study, the
BODT of n-butanoic acid was 46, 48, and 58% after 2, 10, and
30 days, respectively, using a sewage seed. In a screening study
using a sewage seed, n-butanoic acid had a 5-day BODT of
72–78% and a 20-day BODT of 92–99%.
Butyric acid must be separated from strong oxidants, strong
bases, food, and feedstuffs for long-range transport (UN
Hazard Class 8; UN Packing Group III; stable during transport).
It should be stored in a cool, dry, well-ventilated location, away
from any area where fire hazard may be acute. Outside or
detached storage is preferred, separate from oxidizing materials,
heat, oxidizers, and sunlight.
Incompatibilities
Forms explosive mixture with air.Incompatible with sulfuric acid, caustics, ammonia, aliphatic amines, isocyanates, strong oxidizers, alkylene oxides, epichlorohydrin.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Butyric Acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 7621 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
Related articles
- Butyric acid: Production, applications and pharmacodynamics
- Butyric acid is a straight-chain alkyl carboxylic acid and its isomer is isobutyric acid (2-methylpropanoic acid). Salts and e....
- Jul 7,2023
- Use of Butyric acid
- Butyric acid is a four-carbon acid with an unpleasant and obnoxious odor, with a butter-fat taste. It is a clear and colorless....
- Dec 3,2021
View Lastest Price from Butyric Acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | N-Butyric Acid
107-92-6
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2023-12-23 | Butyric Acid
107-92-6
|
US $0.00-0.00 / kg | 1kg | 99% | 50000kg | Hebei Kingfiner Technology Development Co.Ltd | |
 |
2023-05-27 | Butyric acid
107-92-6
|
US $10.00 / kg | 1kg | 99.99% | 50000tons | Henan Bao Enluo International TradeCo.,LTD |
-

- N-Butyric Acid
107-92-6
- US $5.00-2.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Butyric Acid
107-92-6
- US $0.00-0.00 / kg
- 99%
- Hebei Kingfiner Technology Development Co.Ltd
-

- Butyric acid
107-92-6
- US $10.00 / kg
- 99.99%
- Henan Bao Enluo International TradeCo.,LTD
107-92-6(Butyric Acid)Related Search:
1of4