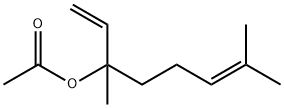
초산 리나릴
|
|
초산 리나릴 속성
- 녹는점
- 85°C
- 끓는 점
- 220 °C(lit.)
- 밀도
- 0.901 g/mL at 25 °C(lit.)
- 증기 밀도
- 6.8 (vs air)
- 증기압
- 0.1 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.453(lit.)
- 인화점
- 194 °F
- 저장 조건
- -20°C
- 용해도
- 클로로포름(약간 용해됨), 메탄올(약간 용해됨)
- 물리적 상태
- 액체
- 색상
- 무색의
- 냄새
- 100.00%에서. 스위트 그린 시트러스 베르가못 라벤더 우디
- ?? ??
- 약초
- 수용성
- 499.8mg/L(25℃)
- JECFA Number
- 359
- Merck
- 14,5496
- BRN
- 1724500
- InChIKey
- UWKAYLJWKGQEPM-LBPRGKRZSA-N
- LogP
- 3.9 at 25℃
- CAS 데이터베이스
- 115-95-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-38 | ||
| 안전지침서 | 26-36-37-24/25 | ||
| 유엔번호(UN No.) | NA 1993 / PGIII | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | RG5910000 | ||
| HS 번호 | 29153900 | ||
| 유해 물질 데이터 | 115-95-7(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 13934 mg/kg | ||
| 기존화학 물질 | KE-11622 |
초산 리나릴 C화학적 특성, 용도, 생산
순도시험
(1) 비중 : 이 품목의 비중은 0.895~0.914이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 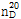 은 1.449~1.457이어야 한다.
은 1.449~1.457이어야 한다.
(3) 용상 : 이 품목 1mL를 70% 에탄올 5mL에 녹일 때, 그 액은 징명하여야 한다.
(4) 산가 : 이 품목의 산가는 향료시험법 중 산가측정법에 따라 시험할 때, 1 이하이어야 한다.
확인시험
이 품목 1mL에 10% 알콜성수산화칼륨용액 5mL를 넣고 수욕 중에서 가열하면 특이한 향기는 없어지고 리나놀 향기가 남는다. 식힌 다음 이에 묽은염산 2mL 및 물 12mL를 넣은 액은 확인시험법 중 초산염 (다)의 반응을 나타낸다.
정량법
이 품목 약 1g을 정밀히 달아 향료시험법 중 에스테르가 및 에스테르함량측정법에 따라 시험한다.
0.5N 알콜성수산화칼륨용액 1mL = 98.14mg C12H20O2
화학적 성질
Linalyl Acetate occurs as its isomer as the main component of lavender oil (30–60%, depending on the origin of the oil), of lavandin oil (25–50%, depending on the species), and of bergamot oil (30–45%). It has also been found in clary sage oil (up to 75%) and in a small amount in many other essential oils. Racemic linalyl acetate is a colorless liquid with a distinct bergamot–lavender odor.
Linalyl acetate is used extensively in perfumery. It is an excellent fragrance material for, among others, bergamot, lilac, lavender, linden, neroli, ylang-ylang, and fantasy notes (particularly chypre). Smaller amounts are used in other citrus products. Since linalyl acetate is fairly stable toward alkali, it can also be employed in soaps and detergents.
출처
Reported found in the essential oils of bergamot, lavender, clary sage and lavandin; also identified among the constituents of the essential oils of Salvia officinalis, petitgrain, sassafras, neroli, lemon, Italian lime, jasmine, Mentha citrata, Mentha aquatica, Thymus mastichina, etc; also reported in abundant quantities in the essential oil from flowers, leaves and stems of Tagetes patula, in the distillate from leaves of Citrus aurantifolia from India, and in the essential oil of Mentha arvensis. Also reported found in citrus peel oils and juices, berries, peach, celery, tomato, cinnamon, clove, nutmeg, pepper, thymus, grape wines, avocado, mushroom, marjoram, mango, cardamom, coriander, gin, origanum, lovage, laurel, myrtle leaf, rosemary, sage and mastic gum oil.용도
Linalyl Acetate ermentative production of medium or short chain length alcohol, esters and/or glucosides by metabolically engineered microorganism.제조 방법
Linalyl acetate is synthesized by two methods:1) Esterification of linalool requires special reaction conditions since it tends to undergo dehydration and cyclization as it is an unsaturated tertiary alcohol. These reactions can be avoided as follows: esterification with ketene in the presence of an acidic esterification catalyst below 30 °C results in the formation of linalyl acetate without any by-products. Esterification can be achieved in good yield, with boiling acetic anhydride, whereby the acetic acid is distilled off as it is formed; a large excess of acetic anhydride must be maintained by continuous addition of anhydride to the still vessel. Highly pure linalyl acetate can be obtained by transesterification of tert-butyl acetate with linalool in the presence of sodium methylate and by continuous removal of the tert-butanol formed in the process.
2) Dehydrolinalool, obtained by ethynylation of 6-methyl-5-hepten-2-one, can be converted into dehydrolinalyl acetatewith acetic anhydride in the presence of an acidic esterification catalyst. Partial hydrogenation of the triple bond to linalyl acetate can be carried out with, for example, palladium catalysts deactivated with lead.
일반 설명
Linalyl acetate is a monoterpene ester mainly found in lavandula essential oil. It is used as a flavoring agent in food industries, as well as a preservative additive in cosmetics and fragrances such as soaps, colognes, perfumes, and skin lotions.색상 색인 번호
Structurally close to linalool, linalyl acetate is the main component of lavender oil and is commonly used in fragrances and toiletries, and in household cleaners and detergents as well. By autoxidation, it leads mainly to hydroperoxides, with a high sensitizing potent.초산 리나릴 준비 용품 및 원자재
원자재
Eucalyptus Citriodara Oil
1,1,3,3,5-펜타메틸-4,6-다이나이트로인단
탄산 칼륨
리나롤
Salvia Root P.E Tanshinone IIA 20%
아세트산(빙초산)
정향유
탄산나트륨(경회)
케텐
준비 용품
초산 리나릴 공급 업체
글로벌( 479)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 81 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |