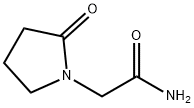
Piracetam
- CAS No.
- 7491-74-9
- Chemical Name:
- Piracetam
- Synonyms
- Nootropil;Pyracetam;2-(2-OXOPYRROLIDIN-1-YL)ACETAMIDE;Geram;CI-871;Axonyl;Cl-781;kt-801;Piramem;Euvifor
- CBNumber:
- CB2354473
- Molecular Formula:
- C6H10N2O2
- Molecular Weight:
- 142.16
- MDL Number:
- MFCD00079246
- MOL File:
- 7491-74-9.mol
| Melting point | 151-152,5 C |
|---|---|
| Boiling point | 259.72°C (rough estimate) |
| Density | 1.2298 (rough estimate) |
| refractive index | 1.5010 (estimate) |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, soluble in ethanol (96 per cent). It shows polymorphism (5.9). |
| pka | 15.67±0.40(Predicted) |
| color | Crystals from isopropanol |
| Water Solubility | Soluble in water (100mM) |
| InChI | InChI=1S/C6H10N2O2/c7-5(9)4-8-3-1-2-6(8)10/h1-4H2,(H2,7,9) |
| InChIKey | GMZVRMREEHBGGF-UHFFFAOYSA-N |
| SMILES | N1(CC(N)=O)CCCC1=O |
| CAS DataBase Reference | 7491-74-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | ZH516LNZ10 |
| ATC code | N06BX03 |
| NIST Chemistry Reference | Piracetam(7491-74-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39-24/25 |
| WGK Germany | 2 |
| RTECS | UX9660500 |
| HS Code | 29339900 |
| Toxicity | LD50 oral in mouse: 2gm/kg |
Piracetam price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | Y0000288 | Piracetam European Pharmacopoeia (EP) Reference Standard | 7491-74-9 | y0000288 | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2675 | Piracetam Pharmaceutical Secondary Standard; Certified Reference Material | 7491-74-9 | 500MG | $145 | 2024-03-01 | Buy |
| Sigma-Aldrich | P5295 | Piracetam | 7491-74-9 | 25g | $100 | 2024-03-01 | Buy |
| Cayman Chemical | 20755 | Piracetam ≥98% | 7491-74-9 | 5g | $68 | 2024-03-01 | Buy |
| Cayman Chemical | 20755 | Piracetam ≥98% | 7491-74-9 | 10g | $95 | 2024-03-01 | Buy |
Piracetam Chemical Properties,Uses,Production
Overview
Piracetam is a cyclic derivative of the neurotransmitter gamma-aminobutyric acid(GABA), originally marketed in 1971 by UCB Pharma. It was the first “nootropic” drug[1], an agent that acts on cognitive function without causing sedation or stimulation. While the mechanisms of action of piracetam have yet to be fully elucidated, it influences neuronal and vascular functions. Furthermore, vascular effects are peripheral as well as central, meaning that the clinical benefit of piracetam goes beyond its nootropic features. Indeed, piracetam is now indicated for use in vertigo, dyslexia, cortical myoclonus and sickle cell anemia in addition to age-related cognitive disorders. Piracetam has been available for over 40 years. Its efficacy has been recorded in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia, however, the clinical application in these conditions is not yet established. Piracetam also has effects on the vascular system through reducing erythrocyte adhesion to vascular endothelium, hinder vasospasm and facilitate microcirculation[2].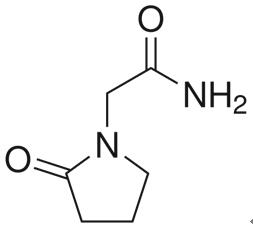
Figure 1 The chemical structure of piracetam
Indication
It is indicated in the case of adult patients suffering from myoclonus of cortical origin, irrespective of aetiology, and should be used in combination with other anti-myoclonic therapies.
Pharmacological effects
Neuronal effects
Piracetam has important effects on neurotransmission that are not limited to any one type of neurotransmitter. It has been shown to influence cholinergic[3,4], serotoninergic[5], noradrenergic[6], and glutamatergic[7] systems. The modulation of these systems by piracetam does not result from direct receptor agonism or antagonism(piracetam has no affinity for these receptors; Ki > 10 μM)[8]. Instead, piracetam appears to increase the number of postsynaptic receptors and or restore the function of these receptors. The effect of piracetam on cholinergic and glutamatergic systems is likely to be particularly relevant to its clinical benefit in cognitive disorders, given the increasing evidence that dysfunction in these systems may be related to cognitive decline[9,10]. Preclinical studies have shown that piracetam appears to offer neuroprotective benefits. This is consistent with the suggestion that interactions between piracetam and membrane lipids may decrease the risk of membrane fusion[11]. Piracetam has been shown to reduce the incidence of animal death following barbiturate overdose, and to protect against morphological changes related to long-term alcohol use[12]. The anticonvulsant action of piracetam has also been documented in animal studies. Administration of piracetam prior to a convulsant stimulus reduces seizure severity in rats prone to audiogenic attacks[13].
Vascular effects
Studies suggest that piracetam exerts a number of effects on erythrocytes, such as decreased adhesion to endothelium[14]. These effects are likely to facilitate movement of erythrocytes through the circulation. Studies have also indicated that piracetam exerts an effect on blood vessels. For example, in vitro, 2 mg/kg piracetam decreased the time taken for rabbit pial vessels to return to normal diameter following a period of induced arteriolar spasm(10.4 vs. 5.1 min for 0.02 and 2 mg/kg of piracetam, respectively)[15]. Piracetam may also influence blood coagulation. In healthy humans, a single dose of piracetam(3.2 to 9.6 g) reduced plasma levels of fibrinogen and von Willebrand factor in a dose-dependent manner by up to 40%[16]. Enhanced cerebral blood flow has been reported following piracetam treatment in hypotensive cats and in humans with acute cerebral ischemia[17,18]. Additionally, piracetam appears to influence microcirculation at the peripheral level. Following treatment with piracetam, renal blood flow was significantly greater in ischemically damaged rat kidneys relative to controls[19], and, in a separate experiment, blood flow significantly increased in the cochlea of guinea pigs without any marked change in blood pressure[20].
Pharmacokinetics
Piracetam is rapidly absorbed. Following oral administration, peak plasma concentrations in fasting subjects are achieved in approximately 30 min[22]. Following a single oral dose of 3.2 g, peak concentration is typically 84 ug/mL. Oral formulations of piracetam are extensively absorbed with a bioavailability close to 100%[22]. No metabolites of piracetam have yet been discovered and the drug is excreted unchanged in the urine by glomerular filtration[21]. While food does not affect the extent of absorption of piracetam, it does decrease the maximal plasma concentration of the drug by 17% and prolong Tmax to 1.5 h. Piracetam crosses blood–brain and placental barriers and is found in all tissues, except adipose tissue. The uptake into the brain is less rapid than into the circulation, and, at nearly 8 h, half-life in cerebrospinal fluid is longer than in plasma(about 5 h)[21].
Mode of action
Although piracetam is a derivative of GABA, its mechanism of action appears to be unrelated to the properties of this neurotransmitter. While the exact mode of action of piracetam is a matter of debate, there is increasing evidence that its underlying effect is to restore cell membrane fluidity. Cell membranes comprise a bilayer of lipid molecules interspersed with protein molecules. These membranes are fluid structures in which the molecules comprising the membrane can diffuse while maintaining this overall arrangement. Membrane fluidity is believed to be important for a number of activities including membrane transport, enzyme activity, chemical secretion, and receptor binding and stimulation[23,24]. Piracetam can have direct interaction with the membrane. The resultant mobile drug–lipid complexes are thought to induce the reorganization of lipids, which may influence membrane function and fluidity[25]. Studies have also demonstrated that piracetam influences membrane fluidity, particularly when normal fluidity is compromised, as is often seen during aging[26]. Several in vitro studies have assessed fluidity during piracetam treatment using anisotropy of membrane-bound DPH[1,6-diphenyl-1,3,5-hexatriene]. Incubation with piracetam restored fluidity in brain membranes of elderly mice with diminished fluidity but had no effect on brain membranes of younger mice with normal fluidity[27]. Similarly, in other studies using in vitro anisotropy techniques, fluidity was restored in the membranes of aged rat and aged human brains following incubation with piracetam[27]. Similar effects were observed in hippocampal membranes from patients with Alzheimer’s disease[28]. This improvement of fluidity coincided with significantly improved avoidance learning[27]. No effect of piracetam on learning or membrane fluidity was found in young rats receiving piracetam. Through restored membrane fluidity, piracetam can promote restored neurotransmission such as glutamatergic and cholinergic systems, enhances neuroplasticity and mediates neuroprotective and anticonvulsant effects at the neuronal level. It has also been found that piracetam can also improves the fluidity of platelet membranes and decreases adhesion of erythrocytes to cell wall as well as reduces vasospasm which in turn improves microcirculation including cerebral and renal blood flow[23,24].
Toxicity
Piracetam is remarkably well tolerated. In preclinical trials, no irreversible toxicity was reported in mice, rats or dogs receiving single oral doses of up to 10 g/kg. In a pooled analysis of 91 double-blind, placebo-controlled studies, hyperkinesia, weight gain, nervousness, somnolence, depression and asthenia were slightly increased with piracetam, although the incidence of each of these events was less than 2%.
Contradictions and drug interactions
Due to its renal clearance, piracetam dose should be adjusted in patients with renal insufficiency and the drug is contraindicated in patients with end-stage renal disease. Piracetam should not be prescribed to patients with cerebral hemorrhage. While reproductive studies in animals have not identified any risk to the fetus, studies in humans have not been conducted and so the use of piracetam in pregnant or lactating women should be avoided. Piracetam is neither metabolized by the liver nor bound to plasma albumin. The potential for drug–drug interactions is, therefore, low. Although piracetam enhances the anticonvulsant effects of carbamazepine, no interactions with sodium valproate have been reported[29]. There are no known interactions of piracetam with any other drugs.
Side effects
Despite that piracetam is regarded as a relatively safe nootropic, some users may still experience side effects and adverse reactions[30]. The severity and number of side effects experienced from piracetam is subject to significant individual variation. One user may not perceive any side effects, while another may notice severe adverse reactions. Of all those reported piracetam side effects, the most common include: nervousness, increased body movements(hyperkinesia), and weight gain. Some rare cases may also include agitation, anxiety, brain fog, cognitive impairment, depression, fatigue, hemocrit/hemoglobin levels change, headaches, hyperkinesia, insomnia, irritability, libido increase, lightheadedness, muscle spasms, nausea, restlessness, shakiness, sleep disturbances, speech impairment, somnolence, sweating, visual change and weakness.
References
- Giurgea C. Vers une pharmacologie de l’activite? inte?grative du cerveau. Tentative du concept nootrope en psychopharmacologie. [Towards an integrative pharmacology of the activity of the brain. Attempt at the nootropic concept in psychopharmacology]. Actual Pharmacol[Paris] 1972;25:115–176.
- Winnicka, K., Tomasiak, M., & Bielawska, A.[2005]. Piracetam--an old drug with novel properties? Acta Poloniae Pharmaceutica, 62(5], 405.
- Mu?ller WE. Age related quantitative and qualitative receptor changes and pharmacological reactivity. In: Racagni G, Mendlewicz J, Eds. Treatment of age-related cognitive dysfunction: Pharmacological and clinical evaluation. Int Acad Biomed Drug Res. Basel: Karger 1992;2:35–40.
- Pilch H, Mu?ller WE. Piracetam elevates muscarinic cholinergic receptor density in the frontal cortex of aged but not of young mice. Psychopharmacology 1988;94:74–78.
- Valzelli L, Bernasconi S, Sala A. Piracetam activity may differ according to the age of the recipient mouse. Int Pharmacopsychiatry 1980;15:150–156.
- Olpe H-R, Steinmann MW. The activating action of vincamine, piracetam and hydergine on the activity of the noradrenergic neurons of the locus coeruleus. Behav Neural Biol 1981;33:249–251.
- Cohen SA, Mu?ller WE. Effects of piracetam on N-methyl-D-aspartate receptor properties in the aged mouse brain. Pharmacology 1993;47:217–222
- Gualtieri F, Manetti D, Romanelli MN, Ghelardini C. Design and study of piracetam-like nootropics, controversial members of the problematic class of cognition-enhancing drugs. Curr Pharm Des 2002;8:125–138.
- Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: A critical perspective. Mech Ageing Dev 2001;122:1–29.
- Terry AV Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 2003; 306:821–827.
- Mingeot-Leclercq M-P, Lins L, Bensliman M, et al. Piracetam inhibits the lipid-destabilising effect of the amyloid peptide Aa? C-terminal fragment. Biochim Biophys Acta 2003;1609:28–38.
- Brandao F, Paula-Barbosa MM, Cadete-Leite A. Piracetam impedes hippocampal neuronal loss during withdrawal after chronic alcohol intake. Alcohol 1995;12:279–288.
- Benesova O. The effects of nootropic drugs on the susceptibility to audiogenic seizures in rats. Act Nerv Super[Praha] 1980;22:192–193.
- Nalbandian RM, Henry RL, Burek CL, et al. Diminished adherence of sickle erythrocytes to cultured vascular endothelium by piracetam. Am J Hematol 1983;15:147–151.
- Reuse-Blom S. Microcirculation of the pial vessels in the rabbit. Acta Cardiol 1979;34:35–36.
- Moriau M, Crasborn L, Lavenne-Pardonge E, Von Frenckell R, Col-Debeys C. Platelet anti-aggregant and rheological properties of piracetam. Arzneimittelforschung 1993;43:110–118.
- Herrschaft H. The effect of piracetam on global and regional cerebral blood flow in acute cerebral ischemia of man. Med Klin 1978;73:195–202.
- Sato M, Heiss WD. Effect of piracetam on cerebral blood flow and somatosensory evoked potential during normotension and hypotensive ischemia in cats. Arzneimittelforschung 1985;35:790–792.
- Gianello P, Janssen T, Chatzopoulos C, et al. Beneficial effect of piracetam on renal blood flow in ischemically injured kidneys in the rat. Transplant Proc 1988;20:914–916.
- Maass B, Soetanto R. Pru?fung der Wirkung von Piracetam auf die Wasserstoff — Clearance am Innenohr [Examination of the effect of piracetam on the hydrogen clearance to the inner ear]. Laryngorhinootologie 1988;67:132–135.
- Gobert JG. Gene?se d’un me?dicament: le piracetam. Me?tabolisation et recherche biochimique. [Genesis of the drug piracetam. Metabolism and biochemical research]. J Pharm Belg 1972;27:281–304.
- Gobert JG, Baltes EL. Availability and plasma clearance of piracetam in man. Farmaco 1977;3:84–91.
- Alberts B, Bray D, Lewis J, et al. Molecular biology of the cell. Chapter 10. 3rd Edition. New York: Garland publishing Inc., 1994.
- Crews FT. Effects of membrane fluidity on secretion and receptor stimulation. Psychopharmacol Bull 1982; 18:135–143.
- Peuvot J, Schank A, Deleers M, Brasseur R. Piracetam-induced changes to membrane physical properties. A combined approach by 31P nuclear magnetic resonance and conformational analysis. Biochem Pharmacol 1995;50:1129–1134.
- Scheuer K, Stoll S, Paschke U, Weigel R, Mu?ller WE. N-methyl-D-aspartate receptor density and membrane fluidity as possible determinants of the decline of passive avoidance performance in aging. Pharmacol Biochem Behav 1995;50:65–70.
- Mu?ller WE, Koch S, Scheuer K, Rostock A, Bartsch R. Effects of piracetam on membrane fluidity in the aged mouse, rat and human brain. Biochem Pharmacol 1997;53:135–140.
- Eckert GP, Cairns NJ, Mu?ller WE. Piracetam reverses hippocampal membrane alterations in Alzheimer’s disease. J Neural Transm 1999;106:757–761.
- Brown P, Steiger MJ, Thompson PD, et al. Effectiveness of piracetam in cortical myoclonus. Mov Disord 1993;8:63–68.
- https://mentalhealthdaily.com/2015/12/18/piracetam-side-effects-adverse-reactions-list/
Description
Piracetam is a nootropic agent with an indication as adjunctive treatment for myoclonus of cortical origin, as well as tardive dyskinesia. However, piracetam is not approved by the FDA for any medical use in the USA. Piracetam should not be prescribed in patients with Huntington disease, those with serious kidney problems, or patients who have experienced a brain haemorrhage. Bleeding problems are a further caution to be taken into account. Although there is little evidence for piracetam’s efficacy in terms of long- term benefits for the treatment of mild cognitive impairments, recent studies showed that it is effective in the treatment of cognitive disorders of cerebrovascular and traumatic origins. With regard to piracetam’s behavioural profile, its beneficial effects on lowering depression and anxiety appear to be higher than its positive effects on memory.
Chemical Properties
White crystalline powder. Odorless, slightly bitter taste. Soluble in water, slightly soluble in ethanol, almost insoluble in ether.
Originator
Nootropyl, UCB ,France,1972
Uses
Piracetam has been used to study crystal structure and physicochemical properties of six co-crystals of piracetam. It has also been used to study its in vitro antioxidant properties.
Uses
Piracetam is most commonly used for breath-holding attacks, seizure disorder (epilepsy), antinauseant, dizziness (vertigo), a learning disorder marked by difficulty reading (dyslexia), nootropic, cortical myoclonus, and a movement disorder often caused by antipsychotic drugs (tardive dyskinesia).
Preparation
Prepared by condensing 2-pyrrolidinone with ethyl chloroacetate in the presence of a metal hydride and then converting the ester into an amide with ammonia.
Definition
ChEBI: Piracetam is an organonitrogen compound and an organooxygen compound. It is functionally related to an alpha-amino acid. It is a compound suggested to be both a nootropic and a neuroprotective agent.
Manufacturing Process
2-Pyrrolidone is first reacted with sodium hydride, then with ethyl chloroacetate to give ethyl 2-oxo-1-pyrrolidine acetate.
A solution of 0.3 mol of ethyl 2-oxo-1-pyrrolidine acetate in 300 ml of methanol, saturated with ammonia at 20° to 30°C, is heated at 40° to 50°C for 5 hours, while continuously introducing ammonia. The reaction mixture is evaporated to dryness and the residue recrystallized from isopropanol. 2-Oxo1-pyrrolidineacetamide is obtained in a yield of 86%. MP 151.5° to 152.5°C.
Therapeutic Function
Psychotropic
Biological Activity
Nootropic that displays cognitive enhancing properties. Proposed to enhance neurotransmission via modulation of ion flux; potentiates Na + influx through AMPA receptors. Facilitates efficiency of cholinergic neurotransmission at muscarinic receptors.
Biochem/physiol Actions
Piracetam (2-oxo-1-pyrrolidinyl-acetamide) is a nootropic drug, which enhances memory and facilitates learning. Piracetam also acts as a vasodilator and improves blood flow. It has a potential to treat cognitive impairment in aging, brain injury, memory and balance problems. In addition, piracetam is also used to treat peripheral vascular disease.
Clinical Use
Myoclonus
Side effects
Most common adverse effects are hyperkinesia, irritability, and weight gain; less commonly, patients can report asthenia, depression, and drowsiness.
Safety Profile
Mildly toxic by ingestion. Whenheated to decomposition it emits toxic fumes of NOx.
Metabolism
The plasma half life of piracetam is approximately 5 hours following oral or intravenous administration. The half life in the cerebrospinal fluid was 8.5 hours. The apparent total body clearance is 80-90 mL/min.
storage
Room temperature
Purification Methods
This typical nootropic (Alzheimer) drug modulates Na flux in AMPA receptors and is purified by recrystallisation from isoPrOH. [Gouilaev & Senning Brain Research Rev 19 180 1994.]
Mode of action
Piracetam is a nootropic drug in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. It shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid and is a cyclic derivative of the neurotransmitter γ-aminobutyric acid (GABA). However its mechanism of action differ from that of endogenous GABA. Piracetam has neuroprotective and anticonvulsant properties and is reported to improve neural plasticity. Its efficacy is documented in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia although the clinical application in these conditions is not yet established. Piracetam has effects on the vascular system by reducing erythrocyte adhesion to the vascular endothelium, hindering vasospasms and facilitating microcirculation.
Piracetam Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Xi'an ZB Biotech Co.,Ltd | +8618591943808 | sales01@xazbbio.com | China | 816 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9339 | 58 |
| APOLLO HEALTHCARE RESOURCES | +6596580999 | sales@apollo-healthcare.com.sg | Singapore | 400 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 | market02@senwayer.com | China | 874 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | 2691956269@qq.com | China | 359 | 58 |
| Dorne Chemical Technology co. LTD | +86-13583358881 +86-18560316533 | Ethan@dornechem.com | China | 294 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 878 | 58 |
Related articles
- The synthesis method of Piracetam
- 2-Pyrrolidone acetamide, also known as piracetam, pirroxil, pyracetam, pyramem and 2-pyrrolidinone acetamide, is a derivative ....
- Mar 12,2024
- Piracetam: Pharmacological Effects and Pharmacokinetics
- Piracetam influences neurotransmitters, enhances neuroplasticity, and exhibits vascular effects, supporting its therapeutic po....
- Feb 20,2024
- Piracetam uses and mode of action
- Piracetam is a cyclic derivative of the neurotransmitter gamma-aminobutyric acid(GABA), originally marketed in 1971 by UCB Pha....
- Dec 21,2022
View Lastest Price from Piracetam manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-27 | Piracetam
7491-74-9
|
US $1.00 / g | 1g | 99% | 100kg | Dorne Chemical Technology co. LTD | |
 |
2024-04-27 | Piracetam
7491-74-9
|
US $1.00-9.90 / g | 1g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-26 | Piracetam
7491-74-9
|
US $10.00 / kg | 1kg | 99.8% | 10000ton | Ouhuang Engineering Materials (Hubei) Co., Ltd |
7491-74-9(Piracetam)Related Search:
1of4