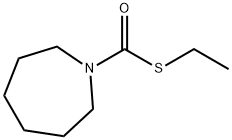
Molinate
- CAS No.
- 2212-67-1
- Chemical Name:
- Molinate
- Synonyms
- G 10;sc998;BUD31;Yalan;Yulan;Felan;Jalan;ordam;HYDRAM;R-4572
- CBNumber:
- CB4768126
- Molecular Formula:
- C9H17NOS
- Molecular Weight:
- 187.3
- MDL Number:
- MFCD00055352
- MOL File:
- 2212-67-1.mol
- MSDS File:
- SDS
| Melting point | <25 °C |
|---|---|
| Boiling point | 202°C (10 mmHg) |
| Density | 1.06 |
| refractive index | 1.5250 (estimate) |
| Flash point | 100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | -1.22±0.20(Predicted) |
| form | Liquid |
| color | Amber |
| Water Solubility | 0.08 g/100 mL |
| BRN | 1239196 |
| LogP | 3.210 |
| CAS DataBase Reference | 2212-67-1(CAS DataBase Reference) |
| EWG's Food Scores | 4-5 |
| FDA UNII | 68N5G08DJQ |
| Proposition 65 List | Molinate |
| NIST Chemistry Reference | Molinate(2212-67-1) |
| EPA Substance Registry System | Molinate (2212-67-1) |
| Pesticides Freedom of Information Act (FOIA) | Molinate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302+H332-H317-H351-H361-H410 |
| Precautionary statements | P201-P273-P280-P301+P312+P330-P304+P340+P312-P308+P313 |
| Hazard Codes | T,N,Xn |
| Risk Statements | 20/22-40-43-48/22-63-50/53-62 |
| Safety Statements | 36/37-46-60-61 |
| RIDADR | 2902 |
| WGK Germany | 3 |
| RTECS | CM2625000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Toxicity | LC50 (96-hour) for bluegill sunfish 29–30 mg/L, goldfish 30 mg/L, mosquito fish 16.4 mg/L, rainbow trout 0.2–1.3 mg/L (Hartley and Kidd, 1987); acute oral LD50 of technical molinate for rats and mice 720 and 795 mg/kg, respectively (Ashton and Monaco, 1991), 501 mg/kg (RTECS, 1985). |
Molinate price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 36171 | Molinate PESTANAL | 2212-67-1 | 100mg | $91.1 | 2024-03-01 | Buy |
| Tocris | 5944 | G10 ≥98%(HPLC) | 2212-67-1 | 50 | $585 | 2021-12-16 | Buy |
| TRC | M486475 | Molinate | 2212-67-1 | 2.5g | $1065 | 2021-12-16 | Buy |
| AK Scientific | J10497 | Molinate | 2212-67-1 | 250mg | $45 | 2021-12-16 | Buy |
| AK Scientific | O973 | S-Ethylazepane-1-carbothioate | 2212-67-1 | 1g | $98 | 2021-12-16 | Buy |
Molinate Chemical Properties,Uses,Production
Uses
Selective herbicide used to control the germination of annual grasses and broadleaved weeds in rice crops.
Definition
ChEBI: Molinate is a member of the class of azepanes that is azepane in which the nitrogen is substituted by an (ethylsulfanyl)carbonyl group, -C(=O)SEt. A thiocarbamate herbicide not approved for use in the U.S. or European Union, it is used control grass weeds in rice paddies. It has a role as an antispermatogenic agent, a herbicide and an agrochemical. It is a member of azepanes and a monothiocarbamic ester.
General Description
Clear liquid with aromatic odor. Non corrosive. Used as an herbicide.
Air & Water Reactions
Water soluble. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
Molinate is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.
Agricultural Uses
Herbicide: Molinate is a selective herbicide used on rice for the control of water grass and other weeds.
Trade name
ARROSOLO®; FELAN®; HIGALNATE®; HYDRAM®; JALAN®; MALERBANE-GIAVONI-L®;ORDAM®; ORDRAM®; R-4572®; RICECO; SAKKIMOL®; STAUFFER R 4,572®; YALAN®; YULAN®
Environmental Fate
Soil. Hydrolyzes in soil forming ethyl mercaptan, carbon dioxide and dialkylamine
(half-life approximately 2–5 weeks) (Hartley and Kidd, 1987). At recommended rates of
application, the half-life of molinate in moist loam soils at 21–27°C is approximately 3
weeks (Humburg et al., 1989). Rajagopal et al. (1989) reported that under flooded conditions,
molinate was hydroxylated at the 3- and 4-position with subsequent oxidation
forming many compounds including molinate sulfoxide, carboxymethyl molinate, hexahydroazepine-
1-carbothioate, 4-hydroxymolinate, 4-hydroxymolinate sulfoxide, hexahydroazepine,
S-methyl hexahydroazepine-1-carbothioate, 4-ketomolinate, 4-hydroxyhexahydroazepine,
4-hydroxy-N-acetyl-hexahydroazepine, carbon dioxide and bound
residues.
Plant. Molinate is rapidly metabolized by plants releasing carbon dioxide and naturally
occurring plant constituents (Humburg et al., 1989).
Photolytic. Molinate in a hydrogen peroxide solution (120 mM) was irradiated by UV
light (l = 290 nm) at 23°C. The major photooxidation products were the two isomers of
2-oxomolinate (20% yield) and s-molinate oxide (5% yield) (Draper and Crosby, 1984).
Half-lives of 180 and 120 hours were observed using one and two equivalents of hydrogen
peroxide, respectively (Draper and Crosby, 1984). Molinate has a UV absorption maximum
at 225 nm and no absorption at wavelengths >290 nm. Therefore, molinate is not expected
to undergo aqueous photolysis under natural sunlight (l = 290 nm). In the presence of
tryptophan, a naturally occurring photosensitizer, molinate in aqueous solution photodegraded
to form 1-((ethylsulfinyl)carbonyl)hexahydro-1H-azepine, S-ethyl hexahydro-2-
oxo-1H-azepine-1-carbothioate and hexamethyleneimine (Soderquist et al., 1977).
Chemical/Physical. Metabolites identified in tap water were molinate sulfoxide, 3- and
4-hydroxymolinate, ketohexamethyleneimine and 4-ketomolinate (Verschueren, 1983).
Metabolic pathway
Juvenile white sturgeon and common carp are exposed to 14C-molinate in a flow-through metabolism system and oxidize molinate to form several products and hydrolyze or conjugate with glutathione (GSH), the sulfoxide, or sulfone. Both fish form a D-glucuronic acid conjugate. The higher toxicity of molinate in common carp may be due to greater bioconcentration, slower depuration, and less efficient metabolic deactivation. In the blood of common carp, molinate is oxidized by erythrocytes to the sulfoxide and possibly the sulfone, then conjugated with GSH or cysteine and cleaved to form mercapturic acid in both erythrocytes and plasma. Conjugation and possible hemoglobin carbamylation occur only after sulfoxidation of molinate. Molinate is distributed uniformly throughout the soil layers, and its degradation products are identified as 2-oxomolinate, 4-oxomolinate, molinate acid, and hexamethyleneimine. In rice plants, 4-hydroxymolinate, 2-oxomolinate, 4-oxomolinate, S-ethyl-N-carboxymethylthiocarbamate, molinate acid, and molinate alcohol are detected. By the soil microorganisms, oxidation of the S-ethyl moiety is considered to be the main pathway, and hydroxy and oxoderivatives on the azepine ring are identified.
Molinate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29823 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +8619930503259 | cherry@crovellbio.com | China | 18421 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50003 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | sales@senfeida.com | China | 23048 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |