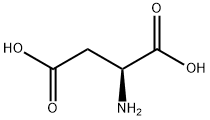
L-아스파라긴 산
|
|
L-아스파라긴 산 속성
- 녹는점
- >300 °C (dec.)(lit.)
- 알파
- 25 º (c=8, 6N HCl)
- 끓는 점
- 245.59°C (rough estimate)
- 밀도
- 1.66
- 굴절률
- 1.4540 (estimate)
- 저장 조건
- Store below +30°C.
- 용해도
- H2O: 5 mg/mL
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 1.99(at 25℃)
- 색상
- 하얀색
- 수소이온지수(pH)
- 2.5-3.5 (4g/l, H2O, 20℃)
- 냄새
- 신맛
- ?? ??
- 냄새 없는
- optical activity
- [α]20/D +24.7±1°, c = 5% in 5 M HCl
- 수용성
- 5 g/L (25 ºC)
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.20
λ: 280 nm Amax: 0.10
- JECFA Number
- 1429
- Merck
- 14,840
- BRN
- 1723530
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제와 호환되지 않습니다.
- InChIKey
- CKLJMWTZIZZHCS-REOHCLBHSA-N
- LogP
- -0.67
- CAS 데이터베이스
- 56-84-8(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36-36/37/38-20/21/22 | ||
| 안전지침서 | 26-24/25-22-36 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | CI9098500 | ||
| F 고인화성물질 | 10 | ||
| TSCA | Yes | ||
| 위험 등급 | IRRITANT | ||
| HS 번호 | 29224995 | ||
| 유해 물질 데이터 | 56-84-8(Hazardous Substances Data) | ||
| 독성 | LD50 intraperitoneal in mouse: 6gm/kg | ||
| 기존화학 물질 | KE-01221 |
L-아스파라긴 산 C화학적 특성, 용도, 생산
순도시험
(1) 용상 : 이 품목 1g을 1N 염산 20mL에 녹인 액은 무색이며 징명하여야 한다.
(2) 액성 : 이 품목의 포화수용액의 pH는 2.5~3.5이어야 한다.
(3) 비선광도 : 이 품목 8g을 정밀히 달아 6N 염산에 녹여 100mL로 하여 이 액의 선광도를 측정하고, 다시 건조물로 환산할 때, 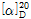 =+24.0~+26.0°이어야 한다.
=+24.0~+26.0°이어야 한다.
(4) 염화물 : 이 품목 0.07g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.2mL에 대응하는 양 이하이어야 한다.
(5) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(6) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
확인시험
(1) 이 품목의 수용액(1→1,000) 5mL에 닌히드린용액(1→50) 1mL를 가하고 수욕상에서 3분간 가열할 때 청자색을 나타낸다.
(2) 이 품목의 1N 염산(1→25) 5mL에 아질산나트륨시액 1mL를 가할 때 기포를 내면서 무색의 가스를 발생한다.
정량법
이 품목 약 0.3g을 정밀히 달아 개미산 6mL에 녹이고 빙초산(비수적정용) 50mL를 가하고 0.1N 과염소산용액으로 적정한다(지시약 : 크리스탈바이올렛․빙초산시액 1mL). 종말점은 액의 자색이 청색을 지나 녹색으로 변하는 점이다. 따로 같은 방법으로 공시험을 한다.
0.1N 과염소산용액 1mL = 13.310mg C4H7NO4
강열잔류물
이 품목의 강열잔류물은 0.1% 이하이어야 한다.
개요
L-Aspartic acid is the L-form of the aspartic acid. It is one of the 20 amino acids that used in the protein synthesis. It is the non-essential amino acids for humans as it can be synthesized in vivo. It is important in the synthesis of other amino acids and some nucleotides, and is a metabolite in the citric acid and urea cycles. In animals, it may be used as a neurotransmitter. It can be chemically synthesize from the diethyl sodium phthalimidomalonate. Currently, almost all the aspartic acids are manufactured in China. Its application include being used as low calorie sweetener (as the part of the aspartame), scale and corrosion inhibitor, and in resins. One of its growing applications is for the manufacturing of biodegradable superabsorbent polymer, polyaspartic acid. It can also be used in fertilizer industry to improve water retention and nitrogen uptake.화학적 성질
Colorless crystals. Soluble in water; insoluble in alcohol and ether. Optically active. dl-aspartic acid.물리적 성질
Solubility 0.5 (25 ℃) g/100 g H2O, pI 2.98, dissociation constants: pK1 2.1, pK2 3.86 (β-COOH), pK3 9.82출처
Dietary sourcesAspartic acid is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans. Aspartic acid is found in :
Animal sources : luncheon meats, sausage meat, wild game
Vegetable sources: sprouting seeds, oat flakes, avocado,
asparagus , young sugarcane, and molasses from sugar beets.
Chemical synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimido malonate, (C6H4(CO)2NC(CO2Et)2).
The major disadvantage of the above technique is that equimolar amounts of each enantiomer are made. Using biotechnology it is now possible to use immobilized enzymes to create just one type of enantiomer owing to their stereo specificity. Aspartic acid is made synthetically using ammonium fumarate and aspartase from E.coli, E.coli usually breaks down the aspartic acid as a nitrogen source but using excess amounts of ammonium fumarate a reversal of the enzyme's job is possible, and so aspartic acid is made to very high yields, 98.7 mM from 1 M.
역사
Aspartic acid was first discovered in 1827 by Plisson, derived from asparagine, which had been isolated from asparagus juice in 1806, by boiling with a base.용도
L-aspartic acid is used as a dietary supplement, it can be blended with minerals to make compounds like potassium aspartate, copper aspartate, manganese aspartate, magnesium aspartate, zinc aspartate and more. Increasing the absorption, and hence utilization potentials, of these minerals via the addition of aspartate induces certain health benefits. Many athletes use L-aspartic acid-based mineral supplements orally to enhance their performance capacities. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. It can help promote a robust metabolism, and is sometimes used to treat fatigue and depression. Aspartic acid is used as a component of parenteral and enteral nutrition. In pharmaceutical agents aspartic acid is used as an ammoniac detoxicating agent, hepar function accelerator and fatigue refresher.정의
ChEBI: The L-enantiomer of aspartic acid.일반 설명
To request documentation for this product, please contact Customer Support and select ‘Product Documentation′. Please note that access to the documentation for this product requires a confidentiality disclosure agreement.위험도
Low toxicity.생물학적 활성
Endogenous NMDA receptor agonist.Safety Profile
Low toxicity by intraperitoneal route. When heated to decomposition emits toxic fumes of NOx.L-아스파라긴 산 준비 용품 및 원자재
원자재
준비 용품
L-아스파라긴 산 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| NINGBO CREATE-BIO ENGINEERING CO.,LTD | +8613906695486 |
13906695486@126.com | China | 1 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 |
admin@juchuangchem.com | China | 387 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 902 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 2880 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15933 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ |
sales03@shyrchem.com | CHINA | 738 | 60 |