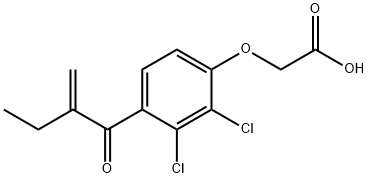
Ethacrynic acid
|
|
Ethacrynic acid 속성
- 녹는점
- 125 °C
- 끓는 점
- 480.0±45.0 °C(Predicted)
- 밀도
- 1.3562 (estimate)
- 저장 조건
- 2-8°C
- 용해도
- DMSO: 용해성20mg/mL, 투명
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 3.50(at 25℃)
- 색상
- 흰색에서 베이지색
- 수용성
- 에탄올, 클로로포름, 에테르, 암모니아, 탄산염 및 메탄올에 용해됩니다. 물에 불용성.
- Merck
- 3717
- CAS 데이터베이스
- 58-54-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22-36/37/38 | ||
| 안전지침서 | 26-36 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AG6600000 | ||
| HS 번호 | 2918992090 | ||
| 유해 물질 데이터 | 58-54-8(Hazardous Substances Data) | ||
| 독성 | LD50 in mice (mg/kg): 176 i.v.; 627 orally (Peck) | ||
| 기존화학 물질 | KE-10151 |
Ethacrynic acid C화학적 특성, 용도, 생산
개요
Ethacrynic acid is a loop diuretic with anticancer activity., It inhibits the Na-K-2Cl (NKCC) cotransporter in duck erythrocytes (IC50 = 0.18 mM) and ATP-dependent chloride uptake in rat renal plasma membrane vesicles when used at a concentration of 0.3 mM., Ethacrynic acid also inhibits glutathione S-transferase P1-1 (GSTP1-1) and GSTA3-3 (IC50s = 4.9 and ~0.4 μM, respectively), and inhibits Wnt/β-catenin signaling in a cell-based reporter assay. It is cytotoxic to primary chronic lymphocytic leukemia cells (IC50 = 8.56 μM), as well as MCF-7, MDA-MB-231, and 4T1 cancer cells (IC50s = 45.53, 39.64, and 25.23 μM, respectively). Ethacrynic acid (250 μg per day) increases tumor growth reduction induced by the EGFR family inhibitors afatinib (Item Nos. 11492 | 21567) or neratinib in a 4T1 murine breast cancer model. Formulations containing ethacrynic acid have been used in the treatment of edema.화학적 성질
White Solid용도
Ethacrynic acid is a powerful diuretic prescribed for edema associated with cardiac insufficiency, renal edema that does not respond to other diuretics, and edema of the brain and lungs.정의
ChEBI: An aromatic ether that is phenoxyacetic acid in which the phenyl ring is substituted by chlorines at positions 2 and 3, and by a 2-methylidenebutanoyl group at position 4. It is a loop diuretic used to treat high blood pressure resulting from diseases such as congestive heart failure, liver failure, and kidney failure. It is also a glutathione S-transferase (EC 2.5.1.18) inhibitor.일반 설명
White solid.공기와 물의 반응
Insoluble in water.반응 프로필
Ethacrynic acid may react vigorously with strong oxidizing agents. Can react exothermically with reducing agents (such as alkali metals and hydrides) to release gaseous hydrogen. May react exothermically with acids. Reacts exothermically with all bases both organic (for example, the amines) and inorganic.화재위험
Ethacrynic acid is probably combustible.Mechanism of action
The mechanism of action of ethacrynic acid appears to be more complex than the simple addition of sulfhydryl groups of the enzyme to the drug molecule. When the double bond of ethacrynic acid is reduced, the resultant compound is still active, although the diuretic activity is diminished. The sulfhydryl groups of the enzyme would not be expected to add to the drug molecule in the absence of the α,β-unsaturated ketone.These compounds are potent high-ceiling diuretics that resemble ethacrynic acid in their mechanism of action. The ethyl ester group represents a pro-drug that can be easily hydrolyzed to the free carboxyl group. As in ethacrynic acid, a 2,3-dichloro substitution is necessary. In addition, a para-hydroxyl group and an unsubstituted aminomethyl group on the benzene ring are highly beneficial. The carbonyl group can be replaced with an ether or sulfide group. These compounds have no ability to add the sulfhydryl groups of the kidney enzymes. The complete mechanism of action of these compounds remains in doubt.
Ethacrynic acid 준비 용품 및 원자재
원자재
헵테인
아세트산 나트륨
Ethyl phenoxyacetate
2,3-DICHLOROPHENOXYACETIC ACID
클로로아세트산
페닐노르말프로필케톤
황산 나트륨
사이클로헥세인
Para-포름알데하이드
다이메틸아민 수화염화물
염화알루미늄
이황화탄소
부티릴클로라이드
에틸브로모아세트산
디에틸에테르
벤젠
염산
중탄산나트륨(중조)
메틸사이클로헥산
2,3-Dichlorophenol
아세트산(빙초산)
메틸알콜
수산화나트륨
디클로로아니솔
Dilute hydrochloric acid
황산
탄소
준비 용품
Ethacrynic acid 공급 업체
글로벌( 146)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 |
mcbio_sales@163.com | China | 2255 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 |
dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 |
support@targetmol.com | United States | 19973 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 49999 | 58 |