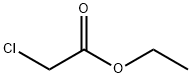
모노클로로초산에틸
|
|
모노클로로초산에틸 속성
- 녹는점
- -26 °C (lit.)
- 끓는 점
- 143 °C (lit.)
- 밀도
- 1.145 g/mL at 25 °C (lit.)
- 증기 밀도
- 4.23 (vs air)
- 증기압
- 10 mm Hg ( 38 °C)
- 굴절률
- n
20/D 1.421(lit.)
- 인화점
- 150 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 12.3g/L
- 물리적 상태
- 액체
- 색상
- 무색의
- 냄새
- 매우 짜증남; 과일 같은; 날카로운
- 폭발한계
- 2.6%(V)
- 수용성
- 20g/L(20℃)
- Merck
- 14,3783
- BRN
- 506455
- Dielectric constant
- 11.6(20℃)
- 안정성
- 안정적인. 산, 염기, 산화제, 환원제와 호환되지 않습니다. 가연성.
- LogP
- 0.940
- CAS 데이터베이스
- 105-39-5(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/24/25-50-10 | ||
| 안전지침서 | 45-61-7/9-16 | ||
| 유엔번호(UN No.) | UN 1181 6.1/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | AF9110000 | ||
| 자연 발화 온도 | 452 °C | ||
| TSCA | Yes | ||
| HS 번호 | 2915 40 00 | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 105-39-5(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 180 mg/kg LD50 dermal Rat 161 mg/kg |
모노클로로초산에틸 C화학적 특성, 용도, 생산
물성
불용성 , 물과 반응.에탄올, 아세톤 및 에틸 에테르에 혼합됨.
알코올, 벤젠, 클로로폼, 에테르에 용해됨.
안전성
건강 3, 화재 2, 반응성 0화재 및 폭발위험 증기는 공기와 결합하여 폭발성 혼합물을 형성할 수 있음; 실내외 하수구내 폭발위험이 있음
"P"로 지정된 물질은 가열되거나 화재시 폭발적으로 중합반응을 일으킬 수 있음
가열시 또는 물로 오염될 경우 용기는 폭발할 수 있음
열이나 화염에 노출시 폭발 위험성이 있음
가열시 팽창이나 분해로 인해 용기의 경우 폭발적으로 파열될 수 있음
고인화성 물질 : 열, 스파크 또는 불꽃에 의해 쉽게 발화됨
증기는 발화원까지 상당한 거리를 이동할 수 있고 역화할 수 있음
금속과 접촉시 인화성 수소가스를 방출할 수 있음
열이나 화염에 노출시 화재 위험성이 있음
소화방법 대형화재 : 일반적인 소화약제를 사용하거나 미세한 물분무로 살수
화학적 성질
Ethyl chloroacetate is a colourless liquid with a pungent, fruity odor.Ethyl chloroacetate has a vapor pressure of 10mmHg at 38 °C (Lewis, 1997). It is insoluble in water, but miscible with alcohol, ether, and acetone (Lide, 1998); it is soluble in benzene (Lewis, 1997). Ethyl chloroacetate readily decomposes in hot water and alkalis (Lewis, 1997).용도
Ethyl chloroacetate is a reagent used in the preparation of 5 member heterocycles. It is used as pharmaceutical and organic intermediate. It is used as a solvent for organic synthesis and the production of pesticides (such as sodium fluoroacetate).제조 방법
Ethyl chloroacetate is synthesized by esterification of chloroacetic acid and ethanol under the catalysis of sulfuric acid. The reaction equation is as follows:ClCH2COOH+C2H5OH[H2SO4]→ClCH2COOC2H5+H2O
Reaction: Add chloroacetic acid, ethanol and benzene into the esterification pot, turn on the stirrer, slowly add sulfuric acid, heat to reflux, continuously steam out the benzene-water azeotrope, and de-esterify the water generated by condensation and separator layering, Benzene is refluxed into the esterification pot, cooled and discharged when there is no more water to steam out, the crude ester is washed with saturated sodium bicarbonate solution and water to neutrality, dried with anhydrous calcium chloride, distilled, and collected The fraction at 144-146°C is the finished product of ethyl chloroacetate, the content is ≥99.0%, and the yield is 85%.
일반 설명
A clear colorless liquid with a pungent odor. Flash point 100°F. Denser than water and insoluble in water. Vapors heavier than air.공기와 물의 반응
Highly flammable. Slow hydrolysis to acidic products will cause slow corrosion of common metals. No hazard involved. [USCG, 1999].반응 프로필
Ethyl chloroacetate is a chlorinated ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.위험도
Strong irritant to eyes.건강위험
Inhalation causes irritation of mucous membrane, headache, and nausea. Contact with liquid causes extreme eye irritation and conjunctivitis; irritates skin if not removed at once. Ingestion causes irritation of mouth and stomach.화재위험
Special Hazards of Combustion Products: Irritating, toxic hydrogen chloride and phosgene may be generated in fires.화학 반응
Reactivity with Water Very slow, not hazardous; Reactivity with Common Materials: Slow hydrolysis to acidic products; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Safety Profile
oison by skin contact and subcutaneous routes. A severe eye irritant. Questionable carcinogen with experimental neoplastigenic data.Flammable liquid; a dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Will react with water or steam to produce toxic and corrosive fumes. Vigorous reaction with sodium cyanide. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of cl-.잠재적 노출
Used to make rodenticides, dyes, and other chemicals. Also used as a military poison운송 방법
UN1181 Ethylchloroacetate, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.Purification Methods
Shake the ester with satutated aqueous Na2CO3 (three times), aqueous 50% CaCl2 (three times) and saturated aqueous NaCl (twice). Dry it with Na2SO4 or MgSO4 and distil it. [Beilstein 2 IV 481.] LACHRYMATORY.비 호환성
May form explosive mixture with air. Incompatible with strong bases; strong acids; reducing agents. Moisture, water, and steam contact forms toxic and corrosive fumes. Violent reaction with oxidizers, alkaline earth metals (barium, calcium, magnesium, strontium, etc.), alkaline metals, sodium cyanide. Attacks metals in the presence of moisture.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.모노클로로초산에틸 준비 용품 및 원자재
원자재
준비 용품
2-PHENYLPROPIONALDEHYDE
CARBOCROMENE HYDROCHLORIDE
N-ethyl perfluorooctylsulfonylaminoacetate
3-(1,1-dimethylethyl)-5-(ethoxycarboxy)-methylthio-1,2,4-triazole
(4-OXO-6,7-DIHYDRO-4H,5H-CYCLOPENTA[4,5]THIENO-[2,3-D]PYRIMIDIN-3-YL)-ACETIC ACID
피록시캄
2-(4-isobutylphenyl)propionaldehyde
GIRARD'S REAGENT P
7-Methoxybenzofuran-2-carboxylic acid
2-오소-1-피리딘아세트아마이드;피라세탐
4-(ACETYLAMINO)PHENOXYACETIC ACID ETHYL ESTER
2-METHYLUNDECANAL
ETHYL 3-(4-ISOBUTYLPHENYL)-3-METHYL GLYCIDATE
메나존
6-Bromoindole-2-carboxylic acid
플루오로아세트산에틸에스테르
1-Boc-3-oxopiperazine
2-Piperazinone
Etomidate
ETHYL 5-BROMOTHIOPHENE-2-CARBOXYLATE
쉬도티오하이드안토인
2-(4-isobutylphenyl)propionaldehyde oxime
SODIUM 3-4-ISOBUTYLPHENYL)-2,3-EPOXYBUTYURATE
Ethyl 6-bromoindole-2-carboxylate
ETHYL 3-PHENYLGLYCIDATE
2-클로로아세트아마이드
5-Bromothiophene-2-carbohydrazide
Cloricromene
(6-ETHYL-4-OXO-4H-THIENO[2,3-D]PYRIMIDIN-3-YL)-ACETIC ACID
6-AMINO-CHROMEN-2-ONE
7-PROPOXY-CHROMEN-2-ONE
나이트로푸란토인
A-METHYL-3-PHENOXYBENZENEACETALDEHYDE
비타민A
TRIETHYL 1,1,2-ETHANETRICARBOXYLATE
Ethyl 2-aminothiazole-5-carboxylate
Methyl 3-methyl-2-butenoate
메틸페닐글리시드산에틸
2-(2-thienyl)propionic acid
(카르복실메틸)트리메틸암모늄 클로라이드 하이드라지드
모노클로로초산에틸 공급 업체
글로벌( 425)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Zouping mingyuan import & export trading co., ltd | +86-0543-2240078 +8618364991597 |
myzhao793@163.com | China | 994 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |